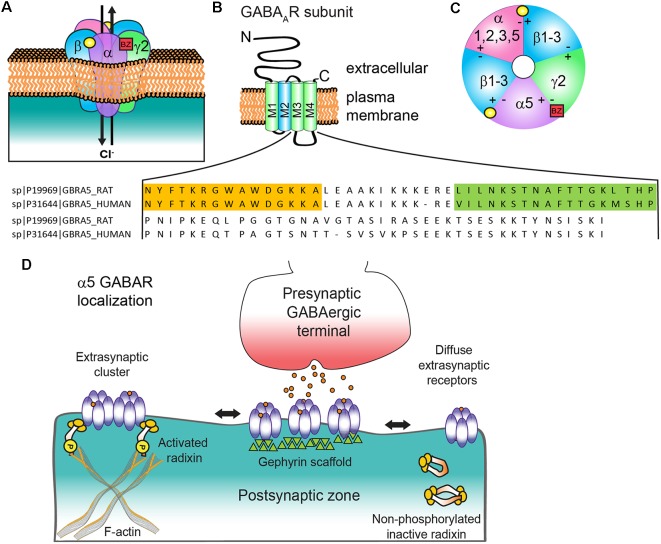
Figure 1.
α5 subunit containing GABA type A receptor (α5 GABAAR) structure and subunit topology. (A) Generic synaptic GABAAR heteropentamer. Binding of the neurotransmitter GABA (yellow circle) at the αβ interface triggers ion channel opening and allows the rapid Cl− influx and membrane hyperpolarization. Benzodiazepines (BZ, red box) bind at the interface of an α1/2/3/5 and γ2 subunit. (B) All subunits have a common topology including an extracellular N-terminal domain (NT), short C-terminal tail (CT), and four transmembrane regions (M1–4) which compose the transmembrane domain. M2 (blue) contributes to formation of the receptor ion channel pore, while the large cytoplasmic domain between M3 and M4 (CD) contains sites for protein interactions and post translational modifications that modulate channel function and/or trafficking: amino acid residue alignment of rat and human α5 CD with radixin binding domain (orange highlighted residues, from Loebrich et al., 2006) and gephyrin interacting region (green highlighted residues, from Brady and Jacob, 2015). (C) α5 GABAAR extracellular representation with potential subunit combinations. (D) Schematic of α5 GABAAR clustering mechanisms at extrasynaptic and synaptic locations with radixin and gephyrin. Phosphorylated radixin interacts with receptors and actin, while with dephosphorylation radixin N-terminal FERM and C-terminal F-actin binding domains interact and form inactive monomers or dimers.