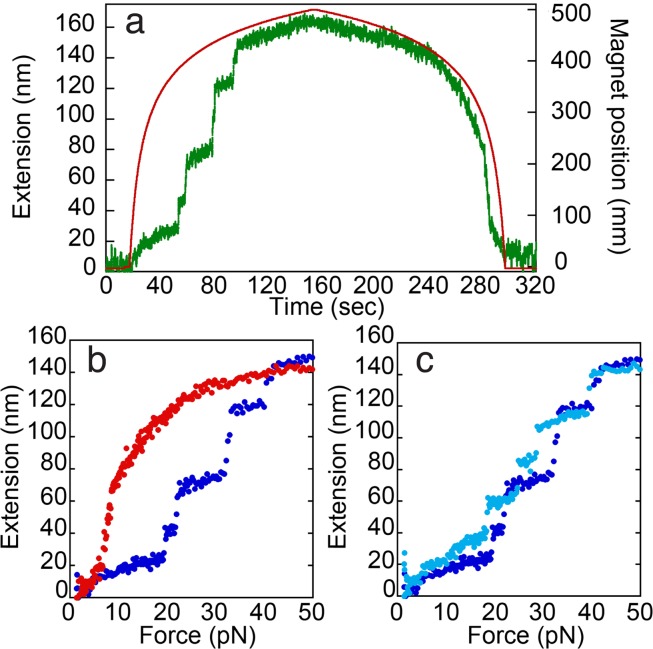
Figure 1.
Force extension curves for spectrin unfolding by magnetic tweezers. (a) A representative trace of the extension (z-position of the beads) and force vs. time data for a typical spectrin pulling experiment. The data were smoothed using a sliding average of window of 10 ms. The magnet is moved relative to the microscope slide at a preprogrammed pattern to exert the desired linear force loading rate on a magnetic bead and the bead position is monitored. After the desired maximum force was reached to unfold the protein, the magnet was retracted at the desired rate and refolding was monitored. (b) Hysteresis is observed between unfolding (blue) and refolding (red). To construct the force vs. extension curve of the unfolding and refolding process the position at each force is averaged and plotted against the force. (c) Force vs. extension data for 2 subsequent unfolding cycles (dark blue first, light blue second) overlay suggesting that the tethered protein refolds properly, and attachment is specific.