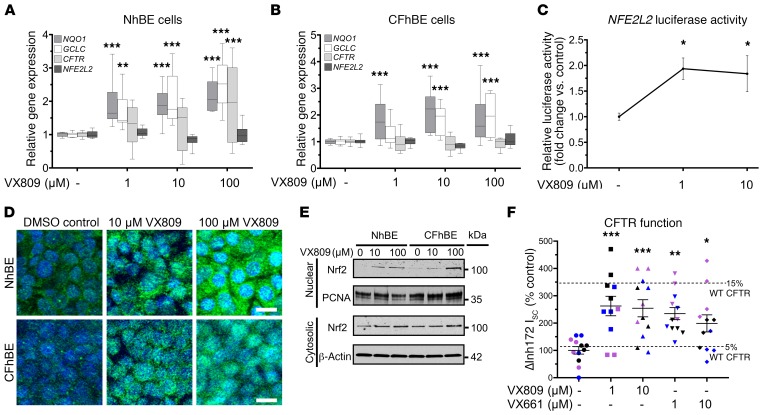
Figure 1. CFTR modulation dose-dependently increases Nrf2 nuclear localization and activity.
(A and B) Gene expression of CFTR, NFE2L2, and Nrf2-regulated genes GCLC and NQO1 in primary NhBE (A) and CFhBE (B) cells, after incubation with DMSO control or the indicated doses of VX809 for 48 hours, determined by real-time quantitative PCR (qPCR). Data presented as fold changes versus untreated cells; calculated from cycle threshold measurements and normalized to 18S rRNA for n = 3 independent experiments and 3 donors per cell type. Data are expressed as box-and-whisker plots. Horizontal bars indicate the median, box borders indicate 25th and 75th percentiles, and whiskers indicate 5th and 95th percentiles. (C) VX809 induces Nrf2-mediated luciferase expression in CFhBE cells. Cells were transiently transfected with a plasmid containing the Nrf2-binding promoter ARE driving firefly and a transfection control Renilla luciferase plasmid, then treated with VX809 (1–10 μM) for 48 hours. Expression in cell lysates was measured by luminometer. Relative activity is firefly/Renilla luciferase activity normalized to total protein, and is expressed as fold change versus DMSO control. Mean ± SEM for n = 7 independent experiments. (D) Representative micrographs of basolateral view of immunofluorescence for Nrf2 (green) or DAPI nuclear staining (blue). Cells were treated with VX809 (10–100 μM) for 48 hours. Scale bars: 10 μm. (E) Western blotting for Nrf2 protein in nuclear and cytosolic fractions of NhBE and CFhBE cells after VX809 treatment for 48 hours; β-actin and proliferating cell nuclear antigen (PCNA) are loading controls. (F) Aggregate short-circuit current data for cells pretreated with VX809 or VX661 (symbols color-coded by donor). Mean ± SEM for 3–4 replicates per condition per donor, from at least 3 unique patient donors, normalized as percentage of donor DMSO control. Dashed lines represent percentage of NhBE control average. For A–C, *P < 0.05, **P < 0.01, ***P < 0.001 vs. DMSO control cells by 1-way ANOVA and Dunnett’s multiple-comparisons test.