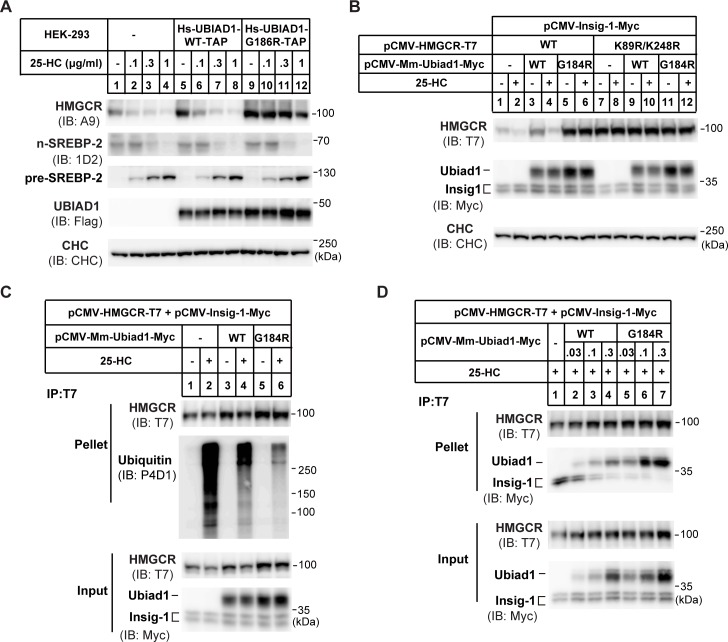
Fig 1. UBIAD1 (G186R) blocks the ubiquitination and degradation of HMGCR.
(A) Effects of human wild-type (WT) UBIAD1 and mutated UBIAD1 (G186R) (equivalent to mouse G184R) on HMGCR protein level. HEK-293 cells stably expressing human (homo sapiens, Hs) WT UBIAD1-TAP and UBIAD1 (G186R)-TAP, respectively, were depleted of sterols in sterol-depleted medium containing 10% LPDS, 1 μM lovastatin and 50 μM mevalonate for 16 hr. Then indicated concentrations of 25-hydroxylcholesterol (25-HC) were added and incubated for 5 hr. Cells were harvested and subjected to immunoblot with antibodies against HMGCR (A9), SREBP-2 (1D2), and UBIAD1 (anti-Flag). Clathrin heavy chain (CHC) was a loading control. TAP: Flag-Protein A tags for tandem affinity purification in S1 Fig. (B) Effects of mouse WT and G184R Ubiad1 on the degradation of the ubiquitin sites mutated (K89R/K248R) HMGCR. CHO-K1 cells were transfected with indicated plasmids, depleted of sterols for 16 hr and treated with 1 μg/ml 25-HC and 10 mM mevalonate (Mev) for 5 hr. Lysates were immunoblotted with indicated antibodies. Mus musculus, Mm. (C) Effects of WT and G184R Ubiad1 on the ubiquitination of HMGCR. CHO-K1 cells were transfected and depleted of sterols, then cells were treated with1 μg/ml 25-HC, 10 mM mevalonate and 20 μM MG-132 for 2 hr before immunoprecipitation. Lysates were immunoprecipitated with anti-T7 antibody coupled agarose, and pellets and inputs were probed for indicated proteins. (D) Interaction between Ubiad1 and HMGCR. CHO-K1 cells were transfected, depleted of sterols and treated with 25-HC for 1 hr, then lysates were immunoprecipitated with anti-T7 antibody coupled agarose. Pellets and inputs were probed with indicated antibodies. The experiments are repeated three times and representative data are shown.