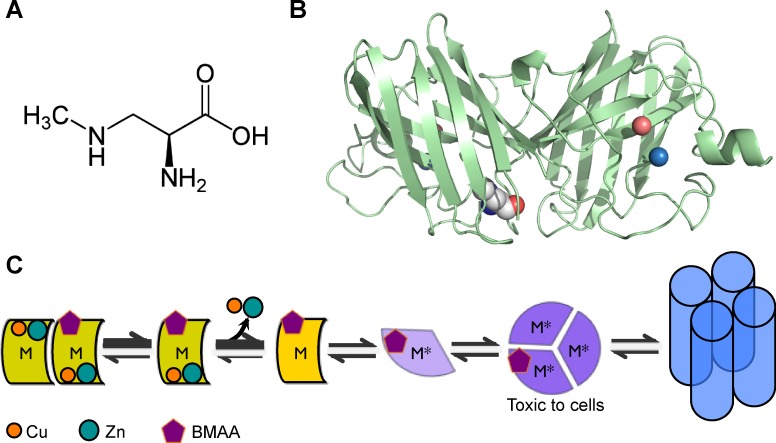
Fig 3. Proposed mechanism of BMAA toxicity in ALS pathology.
(A) Chemical structure of BMAA molecule. (B) Misincorporation of BMAA for serine causes structural rearrangement and strain that propagates to the dimer interface and metal-binding residues. BMAA is show as spheres colored by atom type; copper (orange) and zinc (cerulean) ions are shown as spheres. (C) From left to right: misincorporation of BMAA into SOD1 promotes dimer dissociation and destabilization of the metal-binding sites; metal binding is further destabilized in the monomeric form, leading to metal loss; without metal ions, the SOD1 monomer fold is destabilized misfolds; misfolding promotes oligomerization and the formation of non-native SOD1 trimer, previously shown to be neurotoxic; misfolded SOD1 monomer can also form fibrils observed in ALS patients.