Abstract
The Burkholderia cepacia complex is a group of Gram-negative bacteria that are opportunistic pathogens in immunocompromised individuals, such as those with cystic fibrosis (CF) or chronic granulomatous disease (CGD). Burkholderia are intrinsically resistant to many antibiotics and the lack of antibiotic development necessitates novel therapeutics. Peptide-conjugated phosphorodiamidate morpholino oligomers are antisense molecules that inhibit bacterial mRNA translation. Targeting of PPMOs to the gene acpP, which is essential for membrane synthesis, lead to defects in the membrane and ultimately bactericidal activity. Exploration of additional PPMO sequences identified the ATG and Shine–Dalgarno sites as the most efficacious for targeting acpP. The CF lung is a complex microenvironment, but PPMO inhibition was still efficacious in an artificial model of CF sputum. PPMOs had low toxicity in human CF cells at doses that were antibacterial. PPMOs also reduced the bacterial burden in the lungs of immunocompromised CyBB mice, a model of CGD. Finally, the use of multiple PPMOs was efficacious in inhibiting the growth of both Burkholderia and Pseudomonas in an in vitro model of coinfection. Due to the intrinsic resistance of Burkholderia to traditional antibiotics, PPMOs represent a novel and viable approach to the treatment of Burkholderia infections.
Keywords: antisense, peptide-conjugated phosphorodiamidate morpholino oligomers (PPMO), Burkholaderia cepacia complex (Bcc), artificial sputum, pneumonia
Graphical Abstract
The lung of cystic fibrosis (CF) patients is hypersensitive to infection due to the combined effects of increased mucin, decreased airway surface liquid, and loss of mucociliary action.1,2 Treatment of CF patients is difficult as (i) bacteria readily form biofilms in the increased mucus layer, increasing host and antibiotic resistance,2 (ii) the mucus layer itself is a complex environment that can inactivate many antimicrobial responses and antibiotics (reviewed in ref 3), (iii) bacterial antibiotic resistance is on the rise globally and complicates treatment, and (iv) CF patients are intermittently on antibiotics for much of their life, increasing the selective pressure for resistance. These challenges are in the face of a lack of novel antibiotics in pharmaceutical development.4 Bacterial infections in CF patients requires novel approaches for treatment.
The Burkholderia cepacia complex (Bcc) comprise a group of Gram-negative bacteria, which are found within the environment and as opportunistic pathogens. Bcc generally pose little risk to healthy individuals, but cause disease in immunodeficient patients, such as those with CF or chronic granulomatous disease (CGD).5,6 B. multivorans, B. cepacia, and B. cenocepacia are the most commonly isolated species, and while they account for only three percent of infections in CF patients, they account for significant morbidity and mortality.7–9 Additionally, Bcc are intrinsically resistant to many antibiotics so novel therapies for the treatment of chronic infections are sorely needed.7
Phosphorodiamidate morpholino oligomers (PMOs) are synthetic nucleotides designed to bind to an mRNA of interest and inhibit protein translation, thought to be through steric blockade of the ribosome. PMOs are resistant to enzymatic degradation and contain natural nucleobases connected to synthetic morpholino and phosphorodiamidate intersubunit linkages. PMOs are conjugated to cell-penetrating peptides (PPMOs) to assist in intracellular delivery. We’ve demonstrated previously that PPMOs designed to target acpP are able to inhibit translation most effectively when they are targeted near the Shine–Dalgarno or ATG start site of a gene10 and that PPMOs can be utilized to target essential genes in multiple Gram-negative species including Escherichia coli, Salmonella enterica, Acinetobacter baumannii, Pseudomonas aeruginosa, and Bcc.10–14
The gene for acyl carrier protein was identified as a potential target for antimicrobial development in Burkholderia due to sequence conservation between species and importance for infection in vivo.15 Acyl carrier protein (acpP) is an essential gene which encodes a protein necessary for membrane synthesis.16 Previously, we demonstrated that PPMOs targeted to the ATG of Bcc acpP mRNA inhibited growth and were efficacious in an in vivo septicemia model.11 In this study, we expand upon our previous work, by screening additional PPMOs targeting the −35 to +44 region of the acpP mRNA ATG start site. PPMOs were screened against 39 strains of Bcc, including many clinical strains isolated from CF and CGD patients. We show that our lead PPMO is bactericidal and results in bacterial membrane perturbations. PPMOs are also effective in a complex artificial sputum medium designed to mimic conditions in the CF lung. Importantly, PPMOs have low toxicity at concentrations near their MIC50 and lead PPMOs are efficacious in an immunocompromised mouse pneumonia model. Furthermore, PPMOs can be utilized in combination in vitro to target Bcc and P. aeruginosa simultaneously, which is significant, as CF patients routinely harbor polymicrobial communities in the lung.17–21
RESULTS
Novel PPMO Sequences Targeted to acpP mRNA Are Inhibitory and Bactericidal
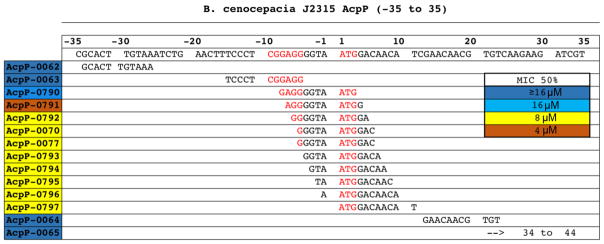
The two originally identified PPMOs were 5′-conjugated (RFF)3RXB PPMOs; the most active of the two was targeted to the acpP start ATG. To identify the best Bcc AcpP-PPMOs we took a two-pronged approach: (i) we designed a series of 3′-conjugated (RFF)3RXB PPMOs across a large region of the acpP gene (AcpP-006Xs and AcpP-0077) from −35 to +44 and (ii) we designed a series of 5′-conjugated (RFF)3RXB PPMOs moving 1 bp at a time (AcpP-079X) from the originally identified sequence (AcpP-0070). Screening with Bcc J2315 revealed that MICs rapidly increased the further the PPMO deviated from the ATG and that the best PPMO, AcpP-0791, spanned both the Shine–Dalgarno and ATG sites (Figure 1). We expanded this screen to include a broad panel of Burkholderia species including clinical isolates from CF and CGD patients and representing Bcc members as well as the non-Bcc B. gladioli and B. glumae. Nine of our PPMOs had significant activity, defined as a MIC50 of less than 8 μM, with AcpP-0791 being the most effective (Figure S1). Interestingly, our panel also included environmentally isolated Burkholderia and the PPMOs were still active in these strains.
Figure 1.
PPMOs are most effective when targeted near the ATG start site. Alignment of AcpP-PPMOs along the acpP gene sequence of B. cenocepacia J2315. The Shine–Dalgarno and ATG start site are displayed in red text and PPMOs target sites are aligned below. The MIC50 values, which is the concentration (μM) that inhibits 50% of strains in Figure S1, are displayed on the ID number (left) using a color scale (right).
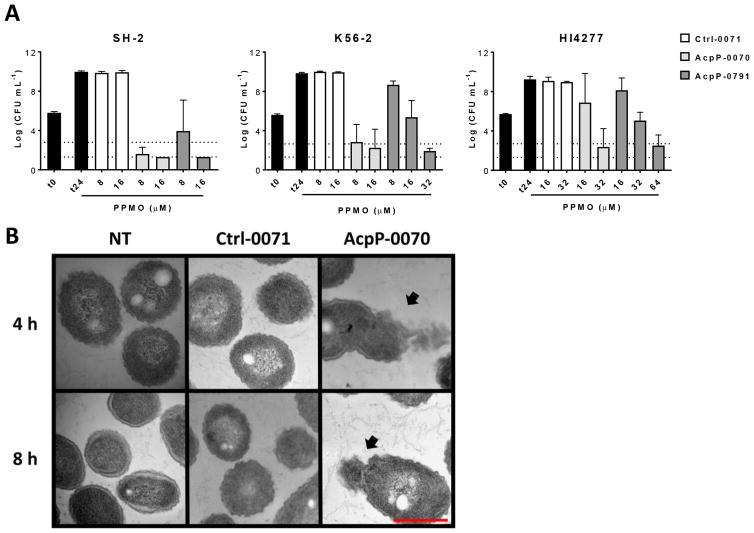
To further demonstrate the effectiveness of these PPMOs, minimum bactericidal concentration (MBC) assays were conducted utilizing PPMO AcpP-0791 compared to our previous lead AcpP-0070 and a control PPMO Ctrl-0071 (Figure 2A). MBCs demonstrate that while both AcpP-0791 and AcpP-0070 are bactericidal in several Bcc strains, acpP-0070 is bactericidal at over 2-fold lower concentration as compared to AcpP-0791 (Figure 2A). Additionally, both PPMOs were able to reach a bactericidal 3-log CFU decrease in a highly antibiotic resistant clinical isolate HI4277,22 albeit requiring higher concentrations (Figure 2A, right). The PPMO target, acpP, is essential for membrane synthesis. Therefore, we hypothesized that PPMO inhibition would lead to membrane disruption. B. multivorans SH-2 was treated with PPMOs and imaged by TEM at four and 8 h post-treatment (Figure 2B). Exposure to AcpP-0070 resulted in membrane perturbation at both time points (Figure 2B, black arrows) as compared to no effect with Ctrl-0071 or no treatment. These data demonstrate that inhibition of acpP translation by PPMO treatment likely leads to cell death due to membrane perturbation due to the lack of AcpP protein.
Figure 2.
AcpP-PPMOs are bactericidal. (A) Minimum bactericidal concentration (MBC) assays for the two lead AcpP-PPMOs in B. multivorans SH-2, B. cenocepacia K56–2, and B. cenocepacia HI4277 after 24 h incubation with indicated PPMOs. Dashed lines in each panel represent the 3-log reduction in CFU (top line) and the limit of detection (bottom line). (B) Transmission electron microscopy of B. multivorans SH-2 after 4 or 8 h incubation with 20 μM Ctrl-0071, AcpP-0070, or no treatment (NT). Black arrows indicate membrane perturbation and red scale bar is 500 nm in all panels.
PPMOs Are Active in Conditions Mimicking the CF Lung
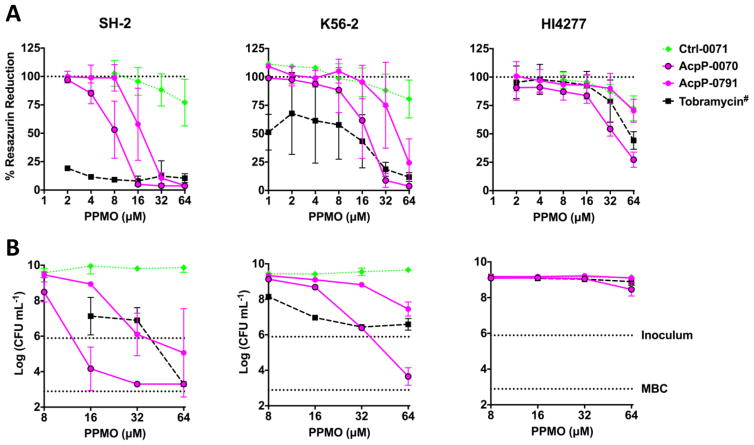
In vitro testing in rich culture media does not recapitulate the complex environment of the lungs, especially in CF patients.23 Artificial sputum medium (ASM) has been developed to more closely approximate the environment of the CF lung, which is high in mucin, DNA, and albumin.23 CLSI testing requires visual inspection of culture media following incubation to detect turbidity. This is not possible in ASM media due to the opacity; therefore alternative methods are used to assess growth. We utilized resazurin, a metabolic dye, which becomes fluorescent following metabolic reduction to resorufin. MBC assays were conducted in ASM with PPMO dosing every 4 h for 24 h (Figure 3). Lead PPMOs AcpP-0791 and AcpP-0070 reduced the amount of metabolically active Bcc (Figure 3A). These data follow the same trend for SH-2 and K56–2 by CFU enumeration (Figure 3B, left and middle). Highly antibiotic resistance Bcc HI4277 was more refractory to treatment, with only the highest concentrations tested able to decrease metabolic activity by 75% (Figure 3A, right), which correlated to a 50% reduction in CFU (Figure 3B, right).
Figure 3.
PPMOs are effective in artificial sputum medium (ASM). Resazurin, a metabolic dye, is reduced by metabolically active bacteria. MBC assays were conducted as in Figure 2A, but with PPMO dosing every 4 h. A sample was removed for CFU enumeration and resazurin was added (100 μM) and incubated at 37 C for 2 h. (A) Metabolic reduction of resazurin was measured with fluorescence spectrometry at 530 nm excitation and 590 nm emission. #Tobramycin was diluted as with PPMO but the highest concentration depicted was 400 μg/mL and the lowest 6.25 μg/mL. The assay was run with at least triplicate experiments and the mean and standard deviation are displayed. (B) CFU were enumerated by serial dilution and plating. The top dotted line represents the inoculum while the bottom represents a 3-log reduction from the inoculum or MBC. These assays were run in duplicate (mean ± SD) with select concentrations to confirm observations with resazurin.
PPMO Therapy Has Low Toxicity at MIC50 Concentrations and Reduces Bcc in a Mouse Model of Pneumonia
PPMOs have previously been shown to have low toxicity in normal human bronchial epithelial cells.24 However, Bcc PPMOs utilize a different cell-penetrating peptide and we wanted to assess toxicity in a relevant CF lung cell, as CF patients are susceptible to Bcc. After 24 h of exposure to 64 μM of PPMO, the highest concentrations of PPMO tested (8- to 16-fold higher than MIC50), there was an 8–40% reduction in viability (Figure 4A). Exposure to the MIC50 concentration (4–8 μM) resulted in no reduction in viability. Having determined that PPMOs had low toxicity at concentrations near the MIC50, we sought to determine if the PPMO efficacy we observed in vitro would be applicable in vivo. We utilized CyBB mice [gp91phox], which are a model of chronic granulomatous disease (CGD),25,26 with our two lead PPMOs. CyBB mice were infected with B. multivorans SH-2 and cotreated with AcpP-0791, Ctrl-0071, or vehicle control (H2O) by intranasal instillation. Coadministration of AcpP-0791 with Bcc resulted in a reduction in CFU burden in the lungs compared to water but not compared to Ctrl-0071 (Figure 4B). AcpP-0070 was included with a second group of mice who received an additional intranasal treatment at 6 h postinfection. Both acpP-0791 and acpP-0070 significantly reduced the CFU burden in the lungs of CyBB mice compared to vehicle treatment; however only AcpP-0070 was able to significantly reduce CFU compared to Ctrl-0071 (Figure 4C). This finding contrasted with our in vitro MIC screening (Figure S1), but was supported by the MBC data indicating that AcpP-0070 is more efficacious (Figure 2A and 3).
Figure 4.
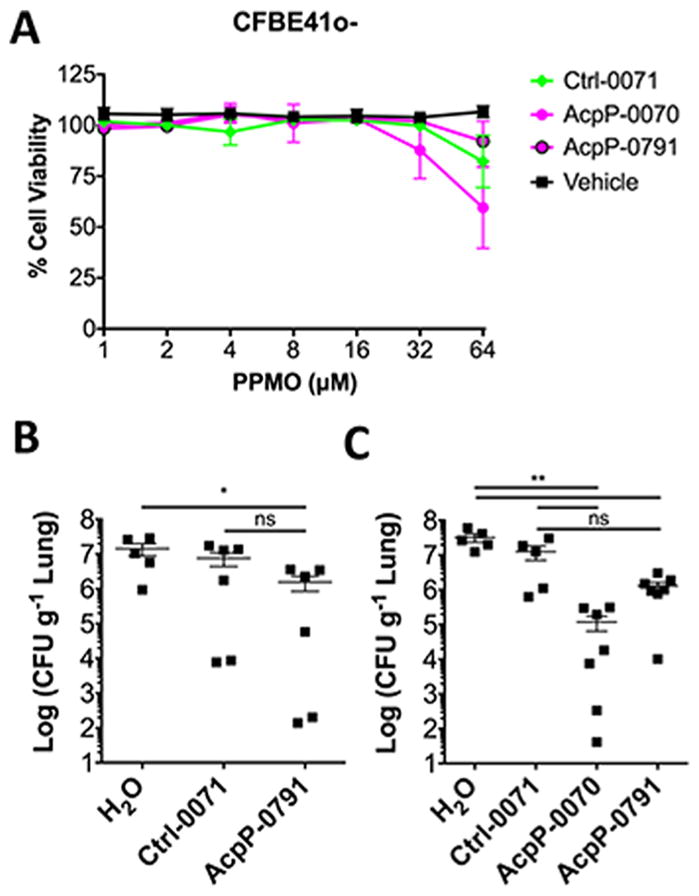
PPMOs have low cytotoxicity and are efficacious in a mouse model of pneumonia. (A) Human CF bronchial epithelial cells (CFHBEs) were seeded onto 96-well tissue cultures plates, stained with resazurin, and incubated for 24 h. Triton X-100, a detergent, was used as a positive cytotoxicity control. (B) CYBB mice, deficient in the phagocyte oxidase, were infected with 6 × 105 CFU of B. multivorans SH-2 by intranasal instillation of 50 μL coadministered with 50 μg of PPMO (2.5 mg/kg). (C) A second group received a second dose at 6 h postinfection in 25 μL (total dose 100 μg or 5 mg/kg). Mice were euthanized at 24 h, and lungs were homogenized for CFU enumeration (n = 5–7). Significance was determined with a Mann–Whitney U test; *p ≤ 0.05, **p ≤ 0.01.
PPMOs Have the Potential to Treat Gram-Negative Coinfections
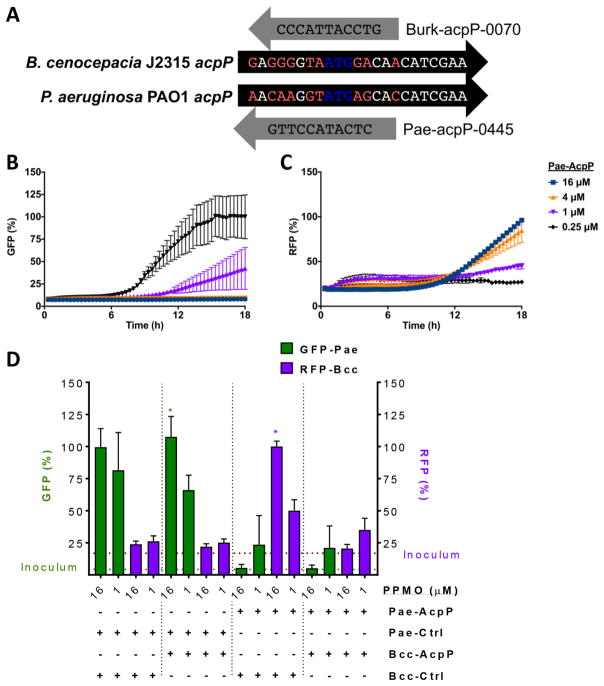
CF patients acquire P. aeruginosa very early in life and progress to acquisition of other pathogens, such as Bcc.9 We have previously demonstrated efficacy of an acpP-targeted PPMO in P. aeruginosa,10 and we therefore hypothesized that utilizing two PPMOs simultaneously would be efficacious in coinfections. Two PPMOs were required because P. aeruginosa acpP and Bcc acpP are not homologous near the ATG start site and different cell-penetrating peptides are required for optimal inhibition (Figure 5A). Utilizing bacteria which each express a different fluorescent marker, we were able to monitor the inhibition of each individual organism during growth (Figure 5B–D). AcpP-0791 (Bcc targeted, Bcc-AcpP) and AcpP-0045 (P. aeruginosa targeted, Pae-AcpP) were combined alone with the relevant Ctrl-PPMO (Bcc-Ctrl or Pae-Ctrl) or together and the Ctrl-PPMOs were combined as controls. With only Pae-AcpP PPMO, Pseudomonas (GFP signal) is inhibited in a dose-dependent manner (Figure 5B), while Burkholderia (RFP signal) increases in a reverse dose-dependent manner (Figure 5C). This is because Pseudomonas has a faster doubling time and outcompetes Burkholderia in the absence of an inhibitor. The Ctrl-PPMO combination led to high GFP signal (Pae) at 18 h but not RFP (Bcc) due to competition (Figure 5D, left). Bcc-AcpP with Pae-Ctrl did not alter the trend (Figure 5D, left middle), as expected, but Pae-AcpP with Bcc-Ctrl resulted in increased RFP signal because the Bcc is able to grow in the absence of Pseudomonas (Figure 5D, right middle). The AcpP combination treatment prevented both the GFP and RFP signals from increasing in a dose-dependent fashion (Figure 5D, right), demonstrating inhibition of both organisms.
Figure 5.
PPMOs retain activity in coinfection models. (A) Alignment of the acpP start site of P. aeruginosa PAO1 and B. cenocepacia J2315 with their respective PPMOs. (B, C) Coinfection model with increasing concentrations of P. aeruginosa AcpP-PPMO demonstrating Pseudomonas GFP signal decreases in a dose-dependent fashion (B), while Bcc RFP signal increases in reverse dose-dependent fashion due to the removal of the competitive organism (C). (D) Coinfection with each AcpP-PPMO combination (only 16 μM or 1 μM data are shown) results in the inhibition of either a single organism (left middle and right middle) or both (right) and the control PPMOs are not inhibitory (left) as assessed at 18 h. *Each fluorophore is normalized to the maximal level captured.
DISCUSSION
We have previously identified two PPMOs, designed to target acpP, with activity against a small panel of Bcc.11 In this manuscript, we further explored potential PPMO sequences targeting acpP and identified AcpP-0791, which spans the ATG and Shine–Dalgarno sites, as the most effective PPMO. The site of conjugation (5′ vs 3′) did not affect potency, which we have observed previously.5,10 The gene target, acpP, is essential for membrane synthesis; therefore PPMO inhibition leads to membrane defects and bactericidal killing. Utilizing our PPMO design tool, both lead AcpP-PPMOs target a region that is 96% conserved within the available Burkholderia genomes (taxon ID 32008). Additionally, the PPMO target is 100% conserved in the three major CF Burkholderia species and the potential biothreats B. mallei and B. pseudomallei. This suggests that a single PPMO would be efficacious against the entire B. cepacia complex.
Standard in vitro testing in rich media is useful for determining antibiotic susceptibility clinically, but it does have limitations. First, MHII medium is rich in divalent cations, which stabilize bacterial membranes,27 but there are numerous host defenses which limit cation availability (e.g., calprotectin). Encouragingly, we have observed enhanced PPMO activity in MH medium (low cation), which is not cation supplemented, suggesting PPMOs would be more efficacious in the nutrient-limiting conditions of the lung (unpublished observations). Second, the lung microenvironment, especially in the CF lung, is considerably more complex than rich media. It is high in mucin, DNA, and albumin, which may increase nonspecific binding.3 Indeed, our testing of PPMOs in ASM media required higher and repeated dosing of PPMOs, presumably due to interactions with medium components. However, PPMOs led to a decrease in bacterial viability in ASM media, approaching a 3-log reduction from inoculum in two strains (Figure 3). The third strain examined in ASM, HI4277 (also designated BCC1616), is a pan-resistant isolate from a clinical outbreak.28 While metabolic activity decreased with PPMO treatment, the CFUs of HI4277 were only reduced by 0.5-log in ASM media; this is despite a MIC of 8–16 μM with our PPMOs in rich medium (Figure S1). This discrepancy may be partially explained by our observations that HI4277 has an intrinsically slow growth rate in liquid media, although it does reach a similar CFU by 24 h.
Mutations in the CF transmembrane regulator lead to multiple cellular changes. We therefore decided to assess PPMO toxicity in CFBE41o- cells that express the most common CF mutation (ΔF508). On the basis of in vitro cellular toxicity assays, PPMOs had low cellular toxicity at concentrations that we anticipate would be clinically achievable doses (Figure 4A and Figure S1). These findings and activity in a biologically relevant media (ASM) led us to assess PPMO activity in a pneumonia mouse model. The CyBB mouse, which is a CGD model, is a useful model as it renders mice susceptible to Burkholderia infections; CGD patients, like CF patients, are susceptible to Burkholderia infections. Coadministration of Bcc and PPMO led to a reduction of bacterial burden in the lungs at 24 h and repeated dosing showed a further decrease in lung burden. Surprisingly, although AcpP-0791 had the lowest MIC50 in vitro, AcpP-0070 had enhanced activity in vivo.
CF patients are susceptible to a wide range of organisms, including multiple pathogenic bacteria, and it is not uncommon for coinfections to occur. We were therefore curious if multiple PPMOs could be used to treat a coinfection. The acpP gene is not conserved between Burkholderia and the other major Gram-negative CF pathogen P. aeruginosa, requiring the use of two PPMOs. The cell-penetrating peptide (RXR)4XB is the most efficacious in P. aeruginosa and (RFF)3RXB is not effective; the opposite is true for Bcc. We demonstrate that PPMOs retained activity in these in vitro coinfection models. We hypothesized that the lack of penetration of the nontargeted PPMO increases the likelihood that targeted PPMO reaches the correct organism and therefore multi-organism targeting is effective.
In this manuscript, we describe the expanded design of acpP PPMOs in Bcc and demonstrate efficacy in a Bcc relevant in vivo infection model. PPMOs targeting acpP near the ATG and Shine–Dalgarno sites appear to be the most effective. Inhibition of a gene essential to membrane synthesis led to membrane defects and was bactericidal in normal and CF-mimicking media. The PPMOs had low toxicity toward human CF bronchial epithelial cells at concentrations near their MIC50’s and reduced bacterial burden in a CGD mouse model of pneumonia. Finally, the use of multiple PPMOs to inhibit multiple pathogenic bacteria represents an effective strategy to combat coinfections. These data all suggest that PPMOs could be a viable alternative to standard antibiotic therapy for the treatment of bacterial infections in CF and CGD patients. Future studies will continue to assess PPMO efficacy in lung models of infection, both acute and chronic. We believe that diseases such as CF are an example of a clinical scenario where narrow spectrum therapy utilizing pathogen-specific PPMOs would be advantageous.
METHODS
Peptide-Conjugated Phosphorodiamidate Morpholino Oligomers (PPMOs)
PMO sequences were designed using a custom webtool (https://qbrc2.swmed.edu/Greenberg/oligonucleotide5.cgi?) that has inputs for a taxon ID, gene, alignment region and oligomer length. We design 11-mer PMOs to target near the translation start sites (NTG), while having the lowest off-target hits in the start sites of other genes. PPMOs were synthesized by Sarepta Therapeutics (Cambridge, MA). PPMO gene targets, sequences, peptide, and peptide-conjugation site (5′ vs 3′) are indicated in Table S1. Control (Ctrl) PPMOs are conjugated to the same peptides and in the same orientation as active PPMOs, however they are not complementary to the gene target.12,29
Bacterial Strains
The Bcc strains used in this study were (i) generous donations from Dr. John LiPuma and (ii) Dr. Joanna Goldberg, (iii) from the American Type Culture Collection, or (iv) clinical isolates from UT Southwestern Medical Center. More information about the sources of our isolates can be found in Table S2. Green fluorescent protein (GFP)-expressing P. aeruginosa PAO130 and dsRed-expressing Bcc K56–231 were previously described.
Bacterial Susceptibility Testing
Minimum Inhibitory Concentrations (MICs) of the PPMOs were determined according to the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method, with minor modifications.32 Briefly, each bacterial strain was diluted to a final concentration of approximately 5 × 105 CFU/mL in cation-adjusted Mueller-Hinton broth (MHII) and the PPMO was serially diluted 2-fold from 16 μM to 0.5 μM in a 96-well tissue culture plate. The plates were then covered with gas permeable membrane strips (MIDSCI, St. Louis, MO) and incubated at 37 °C and 225 rpm for 18–20 h. Optical density was measured at 600 nm using a microtiter plate reader and the lowest dose of PPMO at which the average OD600 measured ≤0.06 was recorded as the MIC. Assays were conducted in triplicate across 39 strains of Bcc to determine the MIC50, MIC75, and MIC90 of each PPMO (e.g., MIC50 is the minimum concentration at which 50% of the strains were inhibited). For select PPMOs, the minimum bactericidal concentration (MBC), the concentration that results in a 3-log reduction in CFU/mL, was determined following the OD600 measurement. Aliquots were removed, serially diluted, and plated for CFU enumeration. If no detectable colonies were observed, the limit of detection was used for statistical analyses.
Transmission Election Microscopy
TEM analysis was performed on B. multivorans SH-2 treated with 20 μM Ctrl-0071 or AcpP-0070 PPMOs. Bacteria were diluted to 1 × 108 CFU/mL, treated with PPMO, and incubated at 37 °C and 225 rpm for 4 h, and 8 h. Nontreated (NT) controls were also fixed at the indicated time points. After incubation, the samples were washed with Hank’s Balanced Salt Solution (HBSS; Life Technologies, Grand Island, NY), resuspended in Karnovsky’s fixative (2% Paraformaldehyde, 2.5% Glutaraldehyde and 0.1 M Sodium Phosphate Buffer), and stored at 4 °C. Samples were processed by the Electron Microscopy Core Facility at UT Southwestern Medical Center and TEM grids were captured on an FEI Tecnai G2 Spirit Biotwin microscope (FEI Company, Hillsboro, OR).
Artificial Sputum Medium Experiments
ASM media was prepared as described previously and nonbiological components were of analytical grade.23,33 Briefly, 10 mg/mL porcine mucin (M1778, Sigma-Aldrich, St. Loius, MO), 10 mg/mL bovine serum albumin (A7906, Sigma-Aldrich), 1.4 mg/mL DNA (D3159 Sigma-Aldrich), 5.9 μg/mL diethylene triamine pentaacetic acid, 5 mg/mL NaCl, 2.2 mg/mL KCl, 5 mg/mL Casamino acids (223120, Becton-Dickinson, Franklin Lakes, NJ), and 1.81 mg/mL Tris base were added sequentially and stirred until complete dissolution and then pH was adjusted to 6.5. The medium was sterilized by autoclaving at 110 °C for 15 min, cooled, and 5 mL/L of egg yolk emulsion (R450290, Thermo Scientific, Waltham, MA) was added aseptically before storing at 4 °C for up to a month.
The standard MIC assay was utilized with the following changes. PPMO was dosed at 0 h as in a standard MIC assay and then every 4 h for 24 h (six doses) in a fixed 1 μL volume. At 24 h the well was diluted 1:1 with Dulbecco’s PBS (DPBS; D8537, Sigma-Aldrich) plus 0.1% Triton X100 (X100, Sigma-Aldrich) and mixed to homogeneity by pipetting. The addition of 0.1% Triton X100 is necessary to disrupt clumps that form in viscous ASM media and has no effect on bacterial viability in media with or without PPMOs. Aliquots were removed and plated for CFU enumeration. Resazurin, a metabolic dye that becomes fluorescent when reduced, was added to the remaining volume at a final concentration of 100 μM and incubated for 2 h at 37 °C. Metabolic capacity was determined spectrophotometrically with excitation at 530 nm and emission at 590 nm. Untreated wells served as positive controls, sterile media as negative control, and tobramycin was included as a comparator to PPMOs. Resazurin assays were conducted in triplicate and CFU determinations were completed in duplicate to demonstrate resazurin accurately assesses burden.
Cytotoxicity Assay
The CF human bronchial epithelial cells CFBE41o- were kindly provided by Dr. Dieter C. Gruenert (San Francisco, CA).34,35 CFBE41o-cells have the cystic fibrosis ΔF508 CFTR mutation and were maintained in MEM with 10% FBS at 37 °C and 5% CO2. Cells were seeded in 96-well tissue culture plates at 1 × 104 per well and allowed to adhere for 24 h. PPMO, vehicle (H2O), or 0.01% Triton X-100 was added in a fixed 1 μL volume and cells were incubated for 24 h. Resazurin was added at a final concentration of 100 μM and incubated for 24 h at 37 °C and measured as above. Viability was calculated as percent of positive control (no treatment), and the assay was conducted in quadruplicate.
Mouse Pneumonia Model
Immunocompromised CyBB mice, which lack the phagocyte oxidase (CGD model), were purchased from (Jackson Laboratories, Bar Harbor, ME). CGD mice are relevant due to the susceptibility of CGD patients to Bcc and because CyBB mice are hyper-susceptible to Bcc infection. All experimental procedures were carried out with the approval from the Institutional Animal Care and Use Committee (IACUC) at UT Southwestern (Protocol #: 2016–101626). Mice, aged 16–18 wks, were anesthetized by isoflurane and infected with 6 × 105 CFU of B. multivorans SH-2 by intranasal instillation of 50 μL coadministered with 50 μg of PPMO or H2O (total dose 50 μg or 2.5 mg/kg). A second group received a second i.n. dose at 6 h postinfection in 25 μL (total dose 100 μg or 5 mg/kg). Mice were euthanized 24 h postinfection and whole lungs were harvested for CFU enumeration. Lungs were weighed and homogenized in 1 mL of DPBS, followed by serial dilution and plating for CFU enumeration. Experiments were conducted in at least duplicate with n = 5–7.
Coinfection In Vitro Experiments
For coinfection experiments, the CLSI susceptibility testing procedure was used with minor alterations. PAO1-GFP and K56–3-dsRed were added at a 1:1 ratio at 5 × 105 CFU/mL. Two PPMOs at a 1:1 ratio were diluted 2-fold as normal. Four PPMOs were required: the AcpP-PPMO (AcpP-0070) and Ctrl-PPMO (Ctrl-0071) for Bcc are described in Tab. S1 and the P. aeruginosa AcpP-PPMO (AcpP-0445) and Ctrl-PPMO (Ctrl-0078) are described in Howard et al., 2017.10 Two controls were necessary as P. aeruginosa PPMOs utilize a different cell-penetrating peptide [(RXR)4XB] compared to Bcc [(RFF)3RXB]. Bcc AcpP-PPMO was combined with the Pseudomonas Ctrl-PPMO and Pseudomonas AcpP-PPMO was combined with the Bcc Ctrl-PPMO for single organism targeting. Both AcpP-PPMOs were combined for dual organism targeting and both Ctrl-PPMOs were combined to assess nonspecific effects. Bacterial growth was monitored spectrophotometrically by each organism’s respective fluorophore (GFP excitation 485 nm emission 528 nm; dsRed excitation 530 nm emission 590 nm) every 15 min for 18 h at 37 °C with orbital shaking.
Supplementary Material
Acknowledgments
All work in the Greenberg laboratory was supported by the National Institute of Allergy and Infectious Diseases (NIAID) [AI105980 to DEG; AI111753 to DEG and BLG; AI007520 to SMD]. We would like to acknowledge Jiwoong Kim for the design and maintenance of the PPMO design webtool and Angela Diehl for her assistance with graphic design.
ABBREVIATIONS
- ASM
artificial sputum media
- Bcc
Burkholderia cepacia complex
- CF
cystic fibrosis
- CGD
chronic granulomatous disease
- CyBB
a mouse strain lacking phagocyte oxidase
- GFP
green fluorescent protein
- MBC
minimum bactericidal concentration
- MH
Mueller-Hinton
- MIC
minimum inhibitory concentration
- PMO
phosphorodiamidate morpholino oligomers
- PPMO
peptide-conjugated phosphorodiamidate morpholino oligomers
- (RFF)3RXB
a small cationic peptide containing arginine, phenylalanine, phenylalanine (3 repeats) with an arginine, 6-aminohexanoic acid, beta-alanine linker
- RFP
red fluorescent protein
- (RXR)4XB
a small cationic peptide containing arginine, 6-aminohexanoic acid, arginine (4 repeats) with a 6-aminohexanoic acid, beta-alanine linker
- TEM
transmission election microscopy
Footnotes
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsinfec-dis.7b00235.
A detailed MIC heatmap of our PPMOs used against clinical Bcc strains (Figure S1), the PPMO sequences and peptide combinations used in this manuscript (Table S1), and the Burkholderia strain information (Table S2) (PDF)
Author Contributions
SMD: MIC’s, MBC’s, experiments in ASM, mouse experiments, coculture experiments, and writing. CRS: mouse experiments and writing. KRMB: TEM and MIC heatmap. CFFS: MBC’s, experiments in ASM. RJ: CFBE toxicity. BLG: experimental guidance and writing. DEG: experimental guidance, writing, and funding.
Notes
The authors declare the following competing financial interest(s): BLG is a consultant to Sarepta Therapeutics and an inventor on numerous patents and patent applications involving PPMOs. DEG receives research support from Sarepta Therapeutics and is an inventor on numerous patent applications involving PPMOs. BLG and DEG receive royalties related to their patents.
References
- 1.Kreda SM, Davis CW, Rose MC. CFTR, mucins, and mucus obstruction in cystic fibrosis. Cold Spring Harbor Perspect Med. 2012;2(9):a009589. doi: 10.1101/cshperspect.a009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23(1):146–58. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 3.Bos AC, Passe KM, Mouton JW, Janssens HM, Tiddens HA. The fate of inhaled antibiotics after deposition in cystic fibrosis: How to get drug to the bug? J Cystic Fibrosis. 2017;16(1):13–23. doi: 10.1016/j.jcf.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368(4):299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg DE, Goldberg JB, Stock F, Murray PR, Holland SM, Lipuma JJ. Recurrent Burkholderia infection in patients with chronic granulomatous disease: 11-year experience at a large referral center. Clin Infect Dis. 2009;48(11):1577–9. doi: 10.1086/598937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipuma JJ. Update on the Burkholderia cepacia complex. Curr Opin Pulm Med. 2005;11(6):528–33. doi: 10.1097/01.mcp.0000181475.85187.ed. [DOI] [PubMed] [Google Scholar]
- 7.Drevinek P, Mahenthiralingam E. Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin Microbiol Infect. 2010;16(7):821–30. doi: 10.1111/j.1469-0691.2010.03237.x. [DOI] [PubMed] [Google Scholar]
- 8.Regan KH, Bhatt J. Eradication therapy for Burkholderia cepacia complex in people with cystic fibrosis. Cochrane Database Syst Rev. 2016;11:CD009876. doi: 10.1002/14651858.CD009876.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2015 Annual Data Report. 2016. [Google Scholar]
- 10.Howard JJ, Sturge CR, Moustafa DA, Daly SM, Marshall-Batty KR, Felder CF, Zamora D, Yabe-Gill M, Labandeira-Rey M, Bailey SM, Wong M, Goldberg JB, Geller BL, Greenberg DE. Inhibition of Pseudomonas aeruginosa by Peptide-Conjugated Phosphorodiamidate Morpholino Oligomers. Antimicrob Agents Chemother. 2017;61(4):e01938-16. doi: 10.1128/AAC.01938-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg DE, Marshall-Batty KR, Brinster LR, Zarember KA, Shaw PA, Mellbye BL, Iversen PL, Holland SM, Geller BL. Antisense phosphorodiamidate morpholino oligomers targeted to an essential gene inhibit Burkholderia cepacia complex. J Infect Dis. 2010;201(12):1822–30. doi: 10.1086/652807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geller BL, Marshall-Batty K, Schnell FJ, McKnight MM, Iversen PL, Greenberg DE. Gene-silencing antisense oligomers inhibit acinetobacter growth in vitro and in vivo. J Infect Dis. 2013;208(10):1553–60. doi: 10.1093/infdis/jit460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilley LD, Hine OS, Kellogg JA, Hassinger JN, Weller DD, Iversen PL, Geller BL. Gene-Specific Effects of Antisense Phosphorodiamidate Morpholino Oligomer-Peptide Conjugates on Escherichia coli and Salmonella enterica Serovar Typhimurium in Pure Culture and in Tissue Culture. Antimicrob Agents Chemother. 2006;50(8):2789–2796. doi: 10.1128/AAC.01286-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geller BL, Deere JD, Stein DA, Kroeker AD, Moulton HM, Iversen PL. Inhibition of Gene Expression in Escherichia coli by Antisense Phosphorodiamidate Morpholino Oligomers. Antimicrob Agents Chemother. 2003;47(10):3233–3239. doi: 10.1128/AAC.47.10.3233-3239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sousa SA, Ramos CG, Almeida F, Meirinhos-Soares L, Wopperer J, Schwager S, Eberl L, Leitao JH. Burkholderia cenocepacia J2315 acyl carrier protein: a potential target for antimicrobials’ development? Microb Pathog. 2008;45(5–6):331–6. doi: 10.1016/j.micpath.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Cronan JE., Jr Polar allele duplication for transcriptional analysis of consecutive essential genes: application to a cluster of Escherichia coli fatty acid biosynthetic genes. J Bacteriol. 1996;178(12):3614–20. doi: 10.1128/jb.178.12.3614-3620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bragonzi A, Farulla I, Paroni M, Twomey KB, Pirone L, Lore NI, Bianconi I, Dalmastri C, Ryan RP, Bevivino A. Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response. PLoS One. 2012;7(12):e52330. doi: 10.1371/journal.pone.0052330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwab U, Abdullah LH, Perlmutt OS, Albert D, Davis CW, Arnold RR, Yankaskas JR, Gilligan P, Neubauer H, Randell SH, Boucher RC. Localization of Burkholderia cepacia complex bacteria in cystic fibrosis lungs and interactions with Pseudomonas aeruginosa in hypoxic mucus. Infect Immun. 2014;82(11):4729–45. doi: 10.1128/IAI.01876-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sibley CD, Surette MG. The polymicrobial nature of airway infections in cystic fibrosis: Cangene Gold Medal Lecture. Can J Microbiol. 2011;57(2):69–77. doi: 10.1139/w10-105. [DOI] [PubMed] [Google Scholar]
- 20.Zemanick ET, Sagel SD, Harris JK. The airway microbiome in cystic fibrosis and implications for treatment. Curr Opin Pediatr. 2011;23(3):319–24. doi: 10.1097/MOP.0b013e32834604f2. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, Young VB, LiPuma JJ. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A. 2012;109(15):5809–14. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones AM, Helm J, Richmond R, Mason-Smith E, Brennan A. Highlights of the North American Cystic Fibrosis Conference 2009. J R Soc Med. 2010;103(Suppl 1):S49–54. doi: 10.1258/jrsm.2010.s11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung C, Naughton S, Turnbull L, Tingpej P, Rose B, Arthur J, Hu H, Harmer C, Harbour C, Hassett DJ, Whitchurch CB, Manos J. Gene expression of Pseudomonas aeruginosa in a mucin-containing synthetic growth medium mimicking cystic fibrosis lung sputum. J Med Microbiol. 2010;59(Pt 9):1089–100. doi: 10.1099/jmm.0.019984-0. [DOI] [PubMed] [Google Scholar]
- 24.Ayhan DH, Tamer YT, Akbar M, Bailey SM, Wong M, Daly SM, Greenberg DE, Toprak E. Sequence-Specific Targeting of Bacterial Resistance Genes Increases Antibiotic Efficacy. PLoS Biol. 2016;14(9):e1002552. doi: 10.1371/journal.pbio.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9(2):202–9. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 26.Sousa SA, Ulrich M, Bragonzi A, Burke M, Worlitzsch D, Leitao JH, Meisner C, Eberl L, Sa-Correia I, Doring G. Virulence of Burkholderia cepacia complex strains in gp91phox−/− mice. Cell Microbiol. 2007;9(12):2817–25. doi: 10.1111/j.1462-5822.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 27.Clifton LA, Skoda MW, Le Brun AP, Ciesielski F, Kuzmenko I, Holt SA, Lakey JH. Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir. 2015;31(1):404–12. doi: 10.1021/la504407v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sass A, Marchbank A, Tullis E, Lipuma JJ, Mahenthiralingam E. Spontaneous and evolutionary changes in the antibiotic resistance of Burkholderia cenocepacia observed by global gene expression analysis. BMC Genomics. 2011;12:373. doi: 10.1186/1471-2164-12-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dryselius R, Nekhotiaeva N, Good L. Antimicrobial synergy between mRNA- and protein-level inhibitors. J Antimicrob Chemother. 2005;56(1):97–103. doi: 10.1093/jac/dki173. [DOI] [PubMed] [Google Scholar]
- 30.Bloemberg GV, O’Toole GA, Lugtenberg BJ, Kolter R. Green fluorescent protein as a marker for Pseudomonas spp. Appl Environ Microbiol. 1997;63(11):4543–51. doi: 10.1128/aem.63.11.4543-4551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vergunst AC, Meijer AH, Renshaw SA, O’Callaghan D. Burkholderia cenocepacia creates an intramacrophage replication niche in zebrafish embryos, followed by bacterial dissemination and establishment of systemic infection. Infect Immun. 2010;78(4):1495–508. doi: 10.1128/IAI.00743-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. 9. Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 33.Sriramulu DD, Lunsdorf H, Lam JS, Romling U. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol. 2005;54(Pt 7):667–76. doi: 10.1099/jmm.0.45969-0. [DOI] [PubMed] [Google Scholar]
- 34.Gruenert DC, Basbaum CB, Widdicombe JH. Long-term culture of normal and cystic fibrosis epithelial cells grown under serum-free conditions. In Vitro Cell Dev Biol. 1990;26(4):411–8. doi: 10.1007/BF02623833. [DOI] [PubMed] [Google Scholar]
- 35.Gruenert DC, Finkbeiner WE, Widdicombe JH. Culture and transformation of human airway epithelial cells. Am J Physiol. 1995;268(3 Pt 1):L347–60. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.