Figure 1. Events and reactions identified through voluntary safety reporting.
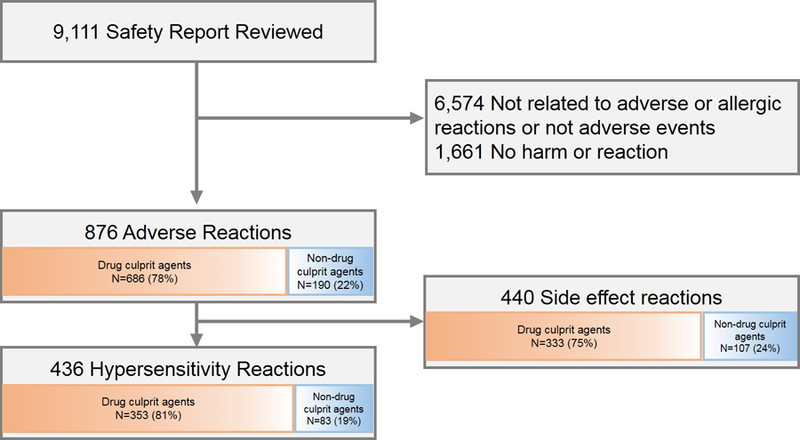
Of 9,111 reviewed event reports, 876 (10%) represented adverse reactions. Adverse reactions were either hypersensitivity reactions (n=436, 50%) or side effect/toxicity reactions (n=440, 50%).
