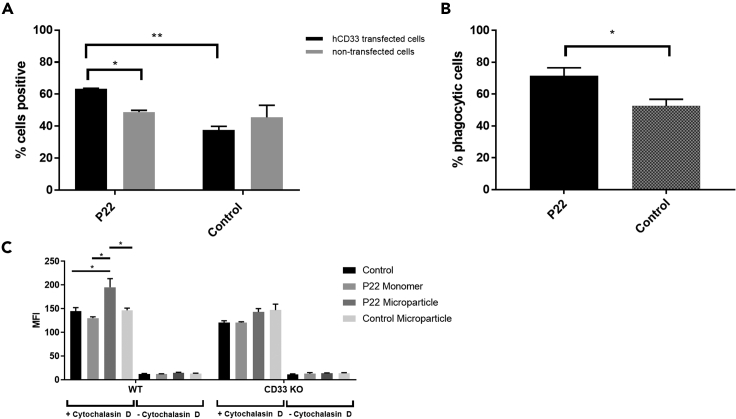
Figure 4.
Effect of P22 on Phagocytosis
The cells were treated with P22 conjugated to microparticles labeled with both streptavidin and fluorescein isothiocyanate (FITC), or with microparticles without P22 conjugated as control.
(A) The percentage of positive cells for FITC microparticles was measured by flow cytometry, demonstrating that P22 preferentially binds to human CD33-transfected cells. Data were obtained in triplicate from n = 3 independent experiments and represented as mean ± SEM. *p < 0.05, **p < 0.01; ANOVA followed by the Tukey test.
(B) Quantification of phagocytic human CD33-transfected cells for Aβ42 pHrodo after being incubated with microparticles reveals an increase of Aβ uptake for cells treated with P22. Data were obtained in triplicate from n = 3 independent experiments and represented as mean ± SEM. *p < 0.05; t test.
(C) Quantification of phagocytic differentiated human THP-1 cells after incubation with P22 monomer, P22 conjugated microparticles, or microparticles without P22 conjugation. Differentiated THP-1 cells were either left untreated or were pre-treated with P22 monomers, P22 conjugated to microparticles, or microparticles alone for 30 min before the addition of E. coli BioParticles. Treatment with P22 conjugated microparticles resulted in a 35% increase in phagocytosis. This increase was not observed in CD33-deficient cells. Co-treatment with the phagocytosis inhibitor, cytochalasin D, blocked phagocytosis in all treatment groups. Data were obtained in triplicate from n = 3 independent experiments and represented as mean ± SEM. *p < 0.05; two-way ANOVA.