Abstract
Background
A major pathway through which obesity increases the risk of cardiometabolic diseases and cancer is by inducing hormonal and metabolic abnormalities, including hyperinsulinemia and altered insulin-like growth factor (IGF) signaling. However, little is known about the influence of lifetime adiposity on the relevant biomarkers.
Objective
The aim of this study was to examine associations of trajectories of body fatness with plasma biomarker concentrations of the insulin-IGF system in 2 large prospective cohorts of US men and women.
Design
Associations between trajectories of body fatness and concentrations of plasma C-peptide, IGF-I, IGF-binding protein (IGFBP) 1, IGFBP-3, and the IGF-I–to–IGFBP-3 molar ratio was examined in 9386 women of the Nurses’ Health Study and 3941 men of the Health Professionals Follow-Up Study. Group-based trajectory modeling was used to create trajectory groups on the basis of self-reported somatotype data at ages 5, 10, 20, 30, and 40 y and body mass index (BMI) at ages 45, 50, 55, and 60 y. We used multivariate linear regression models to examine the associations of trajectories with biomarker concentrations.
Results
Five trajectories of body fatness were identified: “lean-stable,” “lean–moderate increase,” “lean–marked increase,” “medium-stable/increase,” and “medium–marked increase.” Compared with the lean-stable group, the lean–marked increase and medium–marked increase groups had significantly higher concentrations of C-peptide (percentage difference—women: 44% and 73%; men: 27% and 51%) and lower concentrations of IGFBP-1 (women: –61% and –78%; men: –47% and –65%). Adjustment for current BMI attenuated the association to null for the medium–marked increase group, but the lean–marked increase group still had modestly higher concentrations of C-peptide (women: 10%; men: 6%) and lower concentrations of IGFBP-1 (women: –18%; men: –21%) than the lean-stable group.
Conclusions
Adiposity across the life span was associated with higher C-peptide and lower IGFBP-1 concentrations in adulthood. The associations were largely driven by attained adiposity and, to a lesser extent, weight gain in early-middle adulthood. This trial was registered at www.clinicaltrials.gov as NCT03419455.
Keywords: obesity, insulin, C-peptide, IGF-I, IGFBP-1, IGFBP-3
INTRODUCTION
The worldwide prevalence of overweight and obesity is increasing at epidemic rates. In 2014, >1.9 billion adults were overweight, of whom >600 million were obese (1). Excess body fatness increases the risk of several chronic diseases, including type 2 diabetes, cardiovascular disease, and multiple types of cancer (1). Weight gain throughout adulthood has also been associated with increased risk of major chronic diseases and mortality (2–4).
Obesity, particularly the accumulation of visceral abdominal fat, is associated with a variety of metabolic abnormalities, including insulin resistance and hyperinsulinemia (5, 6). Elevated insulin concentrations lead to upregulation of the hepatic production of insulin-like growth factor (IGF) I (7). Insulin and IGF-I are major regulators of energy balance and growth. They exert their effects through interactions with insulin and IGF-I receptors, thereby activating intracellular signaling cascades involved in the regulation of key cellular processes such as cell growth and survival (8). In the circulation, IGF-I interacts with a family of 6 IGF-binding proteins (IGFBPs) that affect its tissue distribution and access to cell receptors (9). Although IGFBP-3 is the major binding protein of IGF-I, IGFBP-1 is proposed to be an important determinant of the acute bioavailability of IGF-I and can be induced by nutrient deprivation and suppressed by insulin (9).
Given that body weight typically increases as a function of age up to age 60–65 y (10), and overweight and obesity in childhood often persist into adulthood (11), a life-course perspective is crucial to better understand the complex relations between obesity, obesity-associated metabolic abnormalities, and future disease risk. Although several observational studies suggest a relation between adult adiposity, hyperinsulinemia, and altered IGF signaling (12), little is known about the lifelong influence of excess body fatness on these biomarkers. Hence, in the current study, we examined the associations between trajectories of body fatness from age 5 to 60 y and plasma C-peptide, a marker of insulin secretion, IGF-I, IGFBP-1, IGFBP-3, and the IGF-I–to–IGFBP-3 molar ratio.
METHODS
Study population
This study used data from 2 large, ongoing US cohort studies, the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS). Detailed information about the 2 cohorts can be found elsewhere (13, 14). In brief, the NHS enrolled 121,700 registered female nurses in 1976, whereas the HPFS enrolled 51,529 male health professionals in 1986. The age range at inclusion was 30–55 and 40–75 y for the NHS and HPFS, respectively. Since enrollment, questionnaires have been administered every 2 y to collect updated lifestyle and medical information. Follow-up rates have been high in both cohorts (95.4% in the NHS and 95.9% in the HPFS up to 2010). Blood samples were collected in 1989–1990 in the NHS and in 1993–1995 in the HPFS. Participants in both studies were mailed a collection kit containing all necessary supplies. Blood samples were returned to the laboratory via overnight courier. Upon receipt, samples were immediately centrifuged, placed in aliquots, and stored in vapor-phase liquid nitrogen freezers at –130°C or below. A total of 32,826 blood samples were returned in the NHS and 18,159 in the HPFS. The blood collection processes for the 2 cohorts have been described in more detail elsewhere (15, 16).
In the current study, we included participants with available biomarker data from previous nested case-control studies of various outcomes within the NHS and HPFS (Supplemental Tables 1 and 2). We excluded participants who had a history of diabetes, cardiovascular disease, and cancer (except for melanoma skin cancer) at blood draw; those who had incomplete body fatness data; those whose biomarker concentrations were considered as outliers; and those who had erroneous records. The final study population consisted of 9386 women and 3941 men (see flowchart in Supplemental Figure 1).
The study was approved by the Institutional Review Board at the Brigham and Women's Hospital and Harvard TH Chan School of Public Health. This trial was registered at www.clinicaltrials.gov as NCT03419455.
Body shape assessment
Body weight and height were recorded at baseline, and then biennially for body weight. In addition, participants were asked to recall their body weight at age 18 y in 1980 in the NHS and at age 21 y in 1986 in the HPFS. Self-reported body weight has been shown to be highly correlated with measured body weight in a validation study including 263 women and men within the NHS and HPFS (correlation coefficient of 0.97 for both sexes) (17). Moreover, a validation study within the NHS II, including 118 women with similar weight characteristics as the overall cohort, showed a correlation coefficient of 0.87 between recalled and measured weight at age 18 y (18). Body weight and height were used to calculate BMI at ages 40, 45, 50, 55, and 60 y. To minimize random variation, we calculated the average BMI at each of these ages ± 3 y. In 1988, participants in the 2 cohorts were asked to recall their body shape in early and middle life with the use of pictorial diagrams (somatotypes), developed by Stunkard et al. (19). Specifically, participants were asked to choose 1 of the 9 somatotypes that best described their body outline at ages 5, 10, 20, 30, and 40 y. The use of recalled somatotypes as an indicator of early-life adiposity has been validated in a previous study, the Third Harvard Growth Study, including 181 women and men aged 71–76 y, of whom ∼50% were lean and 50% were obese in adolescence (20). Validity was assessed on the basis of the correlations between recalled somatotype and BMI measured at approximately the same age. The correlation coefficients for ages 5, 10, and 20 y were 0.60, 0.65, and 0.66 in women and 0.36, 0.66, and 0.53 in men, respectively.
To characterize trajectories of body shape from early throughout middle and late life, we converted participants’ BMI at ages 45, 50, 55, and 60 y to the same scale as the somatotypes (range: 1–9) by applying a linear regression model, in which we linked BMI to somatotype data at age 40. Parameter estimates were used to predict somatotype data later in life on the basis of BMI at the same ages. Hence, we had somatotype data from age 5 to 60 y. Participants who provided somatotype data for at least half of the time points (i.e., 97% in the NHS and 90% in the HPFS) were included in the analysis (Supplemental Figure 1).
Covariate assessment
In both cohorts, detailed lifestyle and medical information has been collected at baseline and then biennially through questionnaires. We included the following covariates in the current study: physical activity, pack-years of smoking, multivitamin use, use of aspirin and other nonsteroidal anti-inflammatory drugs, menopausal status (women only), and use of menopausal hormone therapy (women only). Physical activity was assessed by summing the products of time spent on a variety of activities, mostly recreational or leisure-time physical activity, with the average metabolic equivalent of task for that activity. Pack-years of smoking were calculated as years of smoking times the average number of packs smoked per day.
Recent dietary intake, including alcohol consumption, has been recorded every 2 to 4 y using validated semiquantitative food-frequency questionnaires. As an indicator of the overall dietary pattern, we calculated the Alternative Healthy Eating Index (AHEI) score, which is designed to reflect a healthy dietary pattern emphasizing food choices and macronutrients associated with reduced risk of chronic diseases (21). The dietary score has been associated with a lower risk of several chronic diseases in our cohorts (22).
To capture long-term lifestyle exposures, we calculated cumulative average measurements from baseline to blood draw for physical activity, alcohol consumption, and the AHEI score. Missing information on a questionnaire was carried forward from available information on previous questionnaires.
Validated data on waist and hip circumference were collected in 1986 in the NHS and in 1987 in the HPFS (17). Waist and hip circumferences were used to calculate the waist-to-hip ratio.
Biomarker assessment
Because biomarkers were measured in previous case-control studies in numerous batches over time, differences in mean biomarker concentrations by batch may exist due to variation in analytical factors and long-term storage of blood samples. Therefore, we used a batch correction method developed by Rosner et al. (23) to account for batch variability (see Supplemental Table 3). Batches with a CV of <15% were considered as single batches. For batches with a CV of ≥15%, each assay run was considered as a separate batch. We performed batch correction separately in women and men and used the recalibrated biomarker concentrations for all the analyses.
The IGF-I–to–IGFBP-3 molar ratio, which has been suggested as an indicator of IGF-I bioavailability, was calculated by using the following formula: 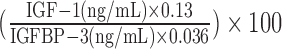 (24). We used the generalized extreme Studentized deviate test to identify and remove outliers (25). The actual number of excluded outliers per biomarker in each cohort is presented in Supplemental Figure 1.
(24). We used the generalized extreme Studentized deviate test to identify and remove outliers (25). The actual number of excluded outliers per biomarker in each cohort is presented in Supplemental Figure 1.
Statistical analysis
Trajectory modeling
Group-based trajectory modeling was used to identify subgroups within each cohort who followed similar evolution of body shape from age 5 to 60 y. The approach has been described in detail previously (3). In brief, longitudinal body shape data were fitted via maximum likelihood as a mixture of multiple latent trajectories in a censored normal model with a polynomial function of age. To determine the optimal number and shape of trajectories, we used a 2-stage approach based on the Bayesian information criterion by first identifying the optimal number of groups when a quadratic form was used for all trajectory groups and then determining the order of the polynomial function specifying the shape of each trajectory. Our final model included 5 groups for each cohort. The model fit statistics including the optimal orders of the polynomial function are presented in Supplemental Tables 4 and 5. The posterior predicted probability for each participant of being a member of each trajectory group was calculated, and participants were assigned to the trajectory group in which their posterior probability of group membership was largest. To test the adequacy of our model, we calculated the mean posterior probability of assignment and the odds of correct classification (OCC) for each group. The OCC is the ratio of odds of correct classification based on the maximum probability classification rule to the odds of correct classification based on random assignment. Both indicators of model adequacy exceeded the recommended threshold (26) (mean posterior probability >0.70 and OCC >5.0) for all trajectories in each cohort. Finally, we named the trajectory groups on the basis of their visual patterns of body shape evolution over age (“lean-stable,” “lean–moderate increase,” “lean–marked increase,” “medium-stable/increase,” and “medium–marked increase”).
Association analysis
Spearman's partial correlation analysis with adjustment for age at blood draw was performed to assess correlations of body shape, BMI, and change in BMI across the lifetime with biomarker concentrations.
Linear regression models were used to examine associations between trajectories of body fatness and plasma biomarker concentrations with the use of the lean-stable group as the reference group. To improve the normality of data distribution, we performed log transformation of the biomarker measurements. Measurement values that became ≤0 after batch correction were set to log(0.01). We built 3 sequential models by adjusting for the following covariates—model 1: age at blood draw; model 2: covariates included in model 1, height, race, physical activity level, alcohol consumption, pack-years of smoking, AHEI dietary score, multivitamin use, regular aspirin/nonsteroidal anti-inflammatory drug use, fasting status, menopausal status, and menopausal hormone therapy; and model 3: covariates included in model 2 and BMI at blood draw.
In addition to the trajectory-biomarker analysis, we performed a joint analysis in which we classified individuals according to the combination of the trajectories and attained BMI levels. To avoid sparse data, we combined the “lean-stable” and “lean–moderate increase” groups as the “lean-stable/moderate increase” group and the “lean–marked increase” and the “medium–marked increase” groups as the “marked increase” group. BMI was categorized into 3 groups: normal weight [BMI (kg/m2) <25.0], overweight (BMI: 25.0–29.9), and obese (BMI ≥30.0). Those with a lean-stable/moderate increase trajectory and normal body weight were treated as the reference in the analysis.
SAS version 9.3 (SAS Institute, Inc.) was used for all analyses. All of the statistical tests were 2-sided, and P values <0.05 were considered significant.
Results
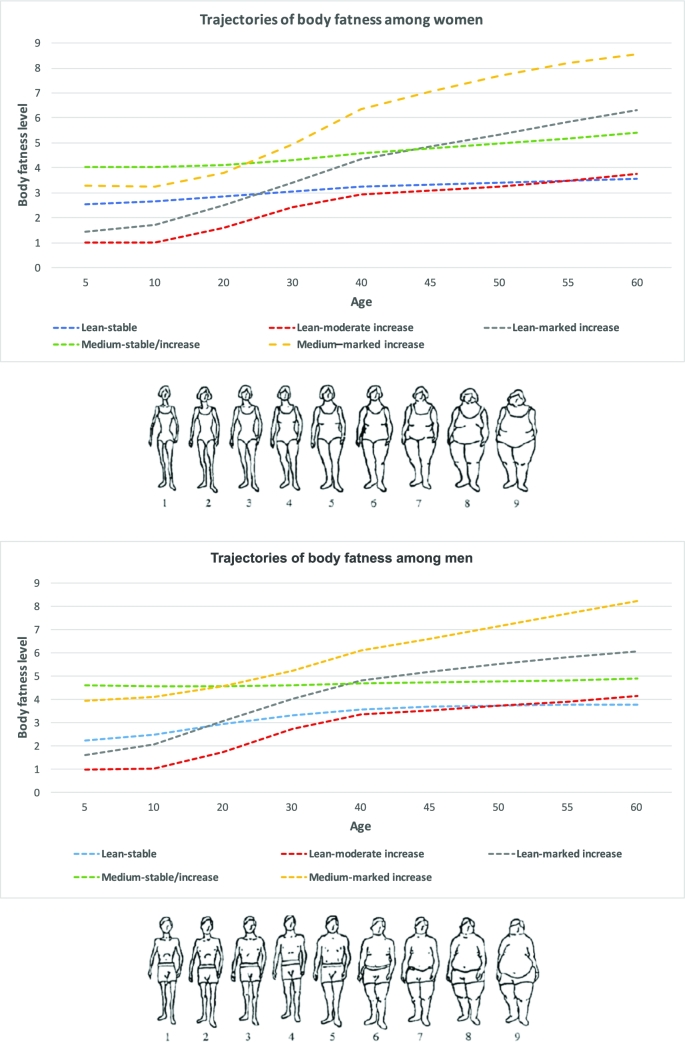
With the use of group-based trajectory modeling, we identified 5 heterogeneous trajectory groups of body fatness from age 5 to 60 y among 9386 women in the NHS and 3941 men in the HPFS. Figure 1 shows the estimated mean body fatness level in each trajectory group as a function of age. Thirty-four percent of women and 29% of men had a lean, albeit slightly increasing, body weight over age (lean-stable group); 23% of women and 19% of men started lean and then experienced a moderate weight gain (lean–moderate increase group); 19% of women and 25% of men started lean and then experienced a substantial increase in body weight (lean–marked increase group); 15% of women and 20% of men started with a medium body shape and then maintained or experienced a small increase in body weight (medium-stable/increase group); and 10% of women and 7% of men started with a medium body shape and then experienced a substantial increase in body weight (medium–marked increase group).
FIGURE 1.
Trajectories of body shape by age in women (top) and men (bottom). With the use of group-based trajectory modeling, we identified 5 heterogeneous trajectory groups of body fatness from age 5 to 60 y among 9386 women in the NHS and 3941 men in the HPFS. Estimated mean body fatness levels in each trajectory group (y axis) as a function of age (x axis) are shown. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
Table 1 shows the basic characteristics of participants at blood draw. As expected, BMI throughout adulthood conformed well to the patterns of the identified trajectories in each group. Moreover, participants in the heavier trajectories had higher waist circumferences and waist-to-hip ratios. The different trajectories were also characterized by distinct lifestyle habits. In general, the lean-stable, lean–moderate increase, and medium-stable/increase groups exercised more and had a higher AHEI score than the lean–marked increase and medium–marked increase groups.
TABLE 1.
Characteristics of study participants at blood draw according to trajectories of body fatness in women (NHS) and men (HPFS)1
| Variable | Lean-stable | Lean–moderate increase | Lean–marked increase | Medium-stable/increase | Medium–marked increase | P 2 |
|---|---|---|---|---|---|---|
| Women | ||||||
| Participants, n (%) | 3184 (34) | 2125 (23) | 1733 (19) | 1433 (15) | 911 (10) | |
| Age, y | 57.6 ± 7.2 | 58.9 ± 6.8 | 56.1 ± 7.1 | 57.1 ± 7.3 | 53.5 ± 6.9 | <0.001 |
| White, n (%) | 3068 (96) | 2014 (95) | 1642 (95) | 1383 (97) | 875 (96) | 0.007 |
| BMI, kg/m2 | ||||||
| At age 18 y | 20.8 ± 2.0 | 19.4 ± 1.8 | 21.2 ± 2.3 | 23.3 ± 2.7 | 24.5 ± 3.5 | <0.001 |
| At age 40 y | 21.2 ± 1.5 | 21.0 ± 1.6 | 24.9 ± 2.2 | 24.8 ± 2.2 | 31.4 ± 4.5 | <0.001 |
| At age 50 y | 22.2 ± 1.7 | 22.2 ± 1.9 | 27.7 ± 2.2 | 26.4 ± 2.2 | 34.8 ± 4.3 | <0.001 |
| At age 60 y | 23.0 ± 2.1 | 23.2 ± 2.3 | 29.6 ± 2.7 | 27.6 ± 2.7 | 36.6 ± 4.3 | <0.001 |
| Height,3 inches | 64.7 ± 2.4 | 64.7 ± 2.4 | 64.4 ± 2.4 | 64.6 ± 2.3 | 64.4 ± 2.5 | <0.001 |
| Waist circumference,4 cm | 74.2 ± 7.0 | 75.5 ± 7.6 | 86.3 ± 9.1 | 82.3 ± 9.6 | 97.2 ± 11.5 | <0.001 |
| Waist-hip ratio4 | 0.76 ± 0.06 | 0.78 ± 0.09 | 0.81 ± 0.11 | 0.79 ± 0.08 | 0.83 ± 0.13 | <0.001 |
| Physical activity,5 MET-h/wk | 17.1 ± 19.1 | 16.6 ± 18.7 | 12.9 ± 14.8 | 14.5 ± 17.6 | 10.7 ± 11.6 | <0.001 |
| Alcohol consumption,5 g/d | 7.8 ± 10.5 | 6.9 ± 9.4 | 4.8 ± 8.0 | 5.9 ± 9.1 | 3.8 ± 7.5 | <0.001 |
| Pack-years of smoking | 12.3 ± 18.6 | 12.3 ± 17.8 | 10.7 ± 17.6 | 13.5 ± 19.8 | 11.1 ± 17.5 | <0.001 |
| Alternative Healthy Eating Index5 | 45.1 ± 8.9 | 44.4 ± 9.0 | 42.5 ± 8.3 | 45.3 ± 8.8 | 41.2 ± 8.3 | <0.001 |
| Multivitamin use, n (%) | 1263 (40) | 847 (40) | 634 (37) | 548 (38) | 313 (34) | 0.01 |
| Aspirin/NSAID use, n (%) | 1486 (47) | 988 (47) | 917 (53) | 743 (52) | 545 (60) | <0.001 |
| C-peptide, ng/mL | 1.6 ± 0.9 | 1.8 ± 1.0 | 2.3 ± 1.1 | 1.9 ± 1.0 | 2.6 ± 1.1 | <0.001 |
| IGF-I, ng/mL | 164 ± 60 | 166 ± 58 | 170 ± 61) | 165 ± 58 | 162 ± 59 | 0.07 |
| IGFBP-1, ng/mL | 43 ± 26 | 39 ± 25 | 24 ± 20 | 32 ± 26 | 15 ± 16 | <0.001 |
| IGFBP-3, ng/mL | 4445 ± 906 | 4549 ± 916 | 4600 ± 983 | 4470 ± 928 | 4545 ± 1095 | <0.001 |
| IGF-I–to–IGFBP-3 molar ratio | 13.4 ± 4.1 | 13.3 ± 4.0 | 13.5 ± 4.1 | 13.4 ± 4.0 | 13.0 ± 3.9 | 0.09 |
| Men | ||||||
| Participants, n (%) | 1146 (29) | 752 (19) | 995 (25) | 772 (20) | 276 (7) | |
| Age, y | 62.1 ± 8.4 | 64.8 ± 7.4 | 62.1 ± 8.4 | 61.1 ± 8.3 | 58.8 ± 7.7 | <0.001 |
| White, n (%) | 1077 (94) | 712 (95) | 946 (95) | 733 (95) | 258 (94) | 0.71 |
| BMI, kg/m2 | ||||||
| At age 21 y | 21.8 ± 1.9 | 21.2 ± 2.0 | 23.3 ± 2.2 | 24.2 ± 2.3 | 26.5 ± 3.0 | <0.001 |
| At age 40 y | 22.6 ± 1.6 | 22.8 ± 1.7 | 25.8 ± 1.8 | 24.7 ± 1.5 | 29.1 ± 2.9 | <0.001 |
| At age 50 y | 23.2 ± 1.4 | 23.6 ± 1.6 | 27.1 ± 1.7 | 25.3 ± 1.7 | 30.8 ± 2.7 | <0.001 |
| At age 60 y | 23.7 ± 1.6 | 24.2 ± 1.9 | 27.8 ± 2.0 | 25.8 ± 2.1 | 32.4 ± 3.1 | <0.001 |
| Height,3 inches | 70.2 ± 2.5 | 70.4 ± 2.8 | 70.4 ± 2.7 | 70.2 ± 2.5 | 70.3 ± 2.7 | 0.11 |
| Waist circumference,4 cm | 90.0 ± 6.8 | 92.5 ± 6.7 | 98.9 ± 7.4 | 94.6 ± 7.4 | 107.7 ± 10.3 | <0.001 |
| Waist-hip ratio4 | 0.92 ± 0.05 | 0.93 ± 0.05 | 0.95 ± 0.05 | 0.93 ± 0.05 | 0.97 ± 0.05 | <0.001 |
| Physical activity,5 MET-h/wk | 35.2 ± 28.2 | 33.8 ± 29.4 | 30.5 ± 24.1 | 34.3 ± 26.3 | 26.3 ± 23.4 | <0.001 |
| Alcohol consumption,5 g/d | 11.9 ± 13.2 | 13.3 ± 15.1 | 13.8 ± 16.0 | 12.8 ± 14.6 | 12.5 ± 15.0 | 0.45 |
| Pack-years of smoking | 9.9 ± 16.2 | 13.6 ± 18.4 | 13.9 ± 18.8 | 12.0 ± 17.2 | 15.0 ± 19.6 | <0.001 |
| Alternative Healthy Eating Index5 | 42.8 ± 9.1 | 41.9 ± 9.2 | 40.1 ± 8.5 | 42.4 ± 8.5 | 38.9 ± 7.9 | <0.001 |
| Multivitamin use, n (%) | 601 (52) | 397 (53) | 479 (48) | 383 (50) | 125 (45) | 0.07 |
| Aspirin/NSAID use, n (%) | 563 (49) | 396 (53) | 554 (56) | 421 (55) | 152 (55) | 0.03 |
| C-peptide, ng/mL | 2.3 ± 1.5 | 2.6 ± 1.6 | 2.9 ± 1.7 | 2.4 ± 1.4 | 3.5 ± 1.9 | <0.001 |
| IGF-I, ng/mL | 156.7 ± 51.3 | 156.5 ± 49.9 | 157.0 ± 50.7 | 157.0 ± 51.0 | 153.3 ± 53.6 | 0.80 |
| IGFBP-1, ng/mL | 24.3 ± 17.4 | 20.5 ± 16.1 | 16.2 ± 14.5 | 21.9 ± 17.8 | 11.0 ± 12.3 | <0.001 |
| IGFBP-3, ng/mL | 3690.0 ± 761.5 | 3713.1 ± 755.8 | 3776.4 ± 812.7 | 3729.7 ± 744.4 | 3697.0 ± 825.6 | 0.20 |
| IGF-I–to–IGFBP-3 molar ratio | 15.3 ± 3.8 | 15.2 ± 3.7 | 15.0 ± 3.8 | 15.2 ± 3.8 | 14.9 ± 3.8 | 0.44 |
1Values are means ± SDs for continuous variables and n (%) for categorical variables. All continuous variables, with the exception of age, were standardized by age at blood draw. HPFS, Health Professionals Follow-Up Study; IGF-I, insulin-like growth factor I; IGFBP, insulin-like growth factor–binding protein; MET-h, metabolic equivalent task hours; NHS, Nurses’ Health Study; NSAID, nonsteroidal anti-inflammatory drug.
2Kruskal-Wallis test was used for continuous variables and chi-square test was used for categorical variables.
3One inch equals 2.54 cm.
4Waist and hip circumferences were assessed in 1986 in the NHS and in 1987 in the HPFS.
5Cumulative average measurements at blood draw.
Table 2 presents the age-adjusted partial correlation coefficients between plasma biomarkers and measures of body fatness throughout life. In general, late-life body fatness (BMI at age 40–60 y) and change in BMI from age 18 or 21 to 60 y were positively correlated to C-peptide concentrations and negatively correlated to IGFBP-1 concentrations in women and men. In contrast, no or weak associations were observed between early-life body fatness (body shape at ages 5 and 10 y and BMI at age 18 or 21 y) and plasma biomarker concentrations.
TABLE 2.
Age-adjusted Spearman correlation coefficients of plasma biomarker concentrations with body shape and BMI across the life span in women (NHS) and men (HPFS)1
| Body shape (somatotype) | BMI | ||||||
|---|---|---|---|---|---|---|---|
| Age 5 y | Age 10 y | Age 18/21 y2 | Age 40 y | Age 50 y | Age 60 y | Change (age 18/21–60 y) | |
| Women | |||||||
| n | 9253 | 9305 | 8958 | 4564 | 8463 | 9288 | 8874 |
| Mean ± SD3 | 2.2 ± 1.3 | 2.5 ± 1.4 | 21.3 ± 2.8 | 23.9 ± 4.2 | 25.2 ± 4.6 | 26.3 ± 5.0 | 5.0 ± 4.6 |
| C-peptide, ng/mL | –0.02*** | 0.01*** | 0.08* | 0.37* | 0.40* | 0.42* | 0.39* |
| IGF-I, ng/mL | –0.04* | –0.05* | –0.05* | –0.04** | –0.02*** | –0.02*** | 0.00*** |
| IGFBP-1, ng/mL | 0.01*** | –0.02*** | –0.08* | –0.44* | –0.45* | –0.46* | –0.43* |
| IGFBP-3, ng/mL | –0.04** | –0.04* | –0.04** | 0.07* | 0.06* | 0.05* | 0.08* |
| IGF-I:IGFBP-3 | –0.02*** | –0.03** | –0.02** | –0.12* | –0.07* | –0.07* | –0.06* |
| Men | |||||||
| n | 3890 | 3923 | 3805 | 396 | 1592 | 3148 | 3057 |
| Mean ± SD3 | 2.4 ± 1.5 | 2.7 ± 1.6 | 22.9 ± 2.6 | 24.6 ± 2.7 | 25.5 ± 2.9 | 25.9 ± 3.2 | 2.9 ± 2.9 |
| C-peptide, ng/mL | –0.07* | –0.04* | 0.05** | 0.28* | 0.30* | 0.29* | 0.26* |
| IGF-I, ng/mL | –0.03** | –0.04** | –0.05** | –0.12** | –0.02*** | –0.01*** | 0.01*** |
| IGFBP-1, ng/mL | 0.04*** | 0.03*** | –0.07** | –0.36* | –0.36* | –0.36* | –0.31* |
| IGFBP-3, ng/mL | –0.03*** | –0.05** | –0.03** | 0.09*** | 0.06** | 0.04*** | 0.07*b |
| IGF-I:IGFBP-3 | –0.02*** | –0.01*** | –0.02*** | –0.20* | –0.05*** | –0.04*** | –0.04*** |
1*P < 0.001; **P < 0.05; ***P ≥ 0.05. HPFS, Health Professionals Follow-Up Study; IGF-I, insulin-like growth factor I; IGFBP, insulin-like growth factor–binding protein; NHS, Nurses’ Health Study.
2BMI at age 18 y for women and at age 21 y for men.
3Values are mean ± SD of somatotype at age 5 and 10 y and BMI (kg/m2) at age 18/21 to 60 y.
Table 3 shows the age-adjusted (model 1), multivariate-adjusted (model 2), and multivariate + current BMI-adjusted associations (model 3) between trajectory groups and plasma biomarker concentrations. Results are presented as percentage differences in biomarker concentrations in each group, with the lean-stable group as the reference. Among women, all trajectory groups had significantly higher C-peptide concentrations compared with the lean-stable group (model 2). The most pronounced difference was observed for the lean–marked increase group (percentage of difference: 44%; 95% CI: 39%, 50%) and medium–marked increase group (percentage of difference: 73%; 95% CI: 65%, 82%). In men, compared with the lean-stable group, C-peptide concentrations were significantly higher in the lean–moderate increase (percentage of difference: 12%; 95% CI: 4%, 20%), lean–marked increase (27%; 95% CI: 19%, 36%), and medium–marked increase (51%; 95% CI: 36%, 67%) groups (model 2). In contrast to C-peptide, plasma IGFBP-1 concentrations were markedly lower in the trajectory groups characterized by heavier body shape. In women, compared with the lean-stable group, IGFBP-1 concentrations were significantly lower in the lean–moderate increase (percentage of difference: –13%; 95% CI: –23%, 0%), lean–marked increase (–61%; 95% CI: –66%, –55%), medium-stable/increase (–43%; 95% CI: –51%, –34%), and medium–marked increase (–78%; 95% CI: –82%, –74%) groups. In men, significant differences were observed in the lean–moderate increase (–17%; 95% CI: –30%, –3%), lean–marked increase (–47%; 95% CI: –54%, –38%), and medium–marked increase (–65%; 95% CI: –72%, –56%) groups. In both women and men, IGF-I concentrations were significantly higher in the lean–moderate increase group compared with the lean-stable group, whereas concentrations were lower in the medium–marked increase group (although not significant for men). No or a very modest difference was observed for IGFBP-3 and the IGF-I to IGFBP-3 molar ratio.
TABLE 3.
Percentage difference (95% CI) in biomarker concentrations between the lean-stable group (reference) and the other trajectory groups in women (NHS) and men (HPFS)1
| Biomarker | Lean-stable | Lean–moderate increase | Lean–marked increase | Medium-stable/increase | Medium–marked increase |
|---|---|---|---|---|---|
| Women | |||||
| C-peptide | |||||
| Model 12 | 0 (reference) | 11 (7, 14) | 47 (42, 52) | 22 (17, 26) | 80 (71, 88) |
| Model 23 | 0 (reference) | 9 (6, 13) | 44 (39, 50) | 20 (16, 25) | 73 (65, 82) |
| Model 34 | 0 (reference) | 9 (5, 12) | 10 (5, 15) | −2 (−6, 3) | −3 (−10, 5) |
| IGF-I | |||||
| Model 12 | 0 (reference) | 4 (1, 6) | 2 (−1, 4) | 0 (−3, 3) | −7 (−10, −4) |
| Model 23 | 0 (reference) | 4 (2, 6) | 1 (−2, 3) | 0 (−2, 3) | −9 (−12, −6) |
| Model 34 | 0 (reference) | 4 (2, 7) | 4 (1, 7) | 3 (−1, 6) | −2 (−7, 3) |
| IGFBP-1 | |||||
| Model 12 | 0 (reference) | −13 (−23, 0) | −63 (−68, −57) | −44 (−52, −35) | −80 (−83, −76) |
| Model 23 | 0 (reference) | −13 (−23, 0) | −61 (−66, −55) | −43 (−51, −34) | −78 (−82, −74) |
| Model 34 | 0 (reference) | −12 (−23, 0) | −18 (−31, −3) | −2 (−17, 15) | 1 (−23, 33) |
| IGFBP-3 | |||||
| Model 12 | 0 (reference) | 3 (1, 4) | 3 (2, 5) | 1 (−1, 2) | 1 (−1, 3) |
| Model 23 | 0 (reference) | 3 (2, 4) | 4 (2, 5) | 1 (−1, 3) | 2 (0, 4) |
| Model 34 | 0 (reference) | 3 (1, 4) | 1 (−1, 3) | −1 (−3, 1) | −3 (−6, 0) |
| IGF-I:IGFBP-3 | |||||
| Model 12 | 0 (reference) | 1 (−1, 3) | −1 (−3, 1) | −1 (−3, 2) | −8 (−10, −6) |
| Model 23 | 0 (reference) | 1 (−1, 3) | −3 (−5, −1) | −1 (−3, 1) | −10 (−12, −8) |
| Model 34 | 0 (reference) | 1 (−1, 3) | 3 (0, 5) | 3 (1, 6) | 1 (−3, 5) |
| Men | |||||
| C-peptide | |||||
| Model 12 | 0 (reference) | 13 (6, 20) | 31 (23, 39) | 3 (−3, 10) | 59 (45, 75) |
| Model 23 | 0 (reference) | 12 (4, 20) | 27 (19, 36) | 2 (−5, 10) | 51 (36, 67) |
| Model 34 | 0 (reference) | 6 (−2, 14) | 6 (−3, 15) | −10 (−17, −3) | −2 (−15, 12) |
| IGF-I | |||||
| Model 12 | 0 (reference) | 4 (0, 8) | −1 (−4, 3) | −1 (−5, 3) | −8 (−13, −3) |
| Model 23 | 0 (reference) | 6 (1, 10) | 2 (−2, 6) | −1 (−5, 3) | −5 (−11, 1) |
| Model 34 | 0 (reference) | 4 (−1, 10) | 2 (−3, 7) | −1 (−6, 4) | −4 (−12, 4) |
| IGFBP-1 | |||||
| Model 12 | 0 (reference) | −22 (−34, −9) | −46 (−53, −37) | −13 (−26, 2) | −67 (−74, −59) |
| Model 23 | 0 (reference) | −17 (−30, −3) | −47 (−54, −38) | −14 (−27, 1) | −65 (−72, −56) |
| Model 34 | 0 (reference) | −11 (−25, 5) | −21 (−35, −6) | 12 (−6, 33) | −12 (−35, 20) |
| IGFBP-3 | |||||
| Model 12 | 0 (reference) | 3 (1, 5) | 2 (0, 4) | 1 (−1, 3) | −3 (−6, 0) |
| Model 23 | 0 (reference) | 3 (1, 6) | 3 (1, 5) | 1 (−1, 3) | −2 (−5, 1) |
| Model 34 | 0 (reference) | 3 (0, 5) | 2 (0, 5) | 0 (−2, 3) | −4 (−8, 0) |
| IGF-I:IGFBP-3 | |||||
| Model 12 | 0 (reference) | 1 (−2, 4) | −2 (−4, 1) | −1 (−4, 1) | −5 (−8, −1) |
| Model 23 | 0 (reference) | 2 (−1, 4) | −1 (−3, 2) | −1 (−3, 2) | −4 (−7, 0) |
| Model 34 | 0 (reference) | 1 (−2, 4) | 0 (−3, 3) | 0 (−3, 3) | −1 (−6, 4) |
1AHEI, Alternative Healthy Eating Index; HPFS, Health Professionals Follow-Up Study; IGF-I, insulin-like growth factor I; IGFBP, insulin-like growth factor–binding protein; MET-h, metabolic equivalent task hours; NHS, Nurses’ Health Study; NSAID, nonsteroidal anti-inflammatory drug.
2Multivariate linear regression model with adjustment for age at blood draw.
3Multivariate linear regression model with adjustment for covariates included in model 1 plus height (continuous), race (white or nonwhite), physical activity (women: <5, 5–11.4, 11.5–21.9, or ≥22 MET-h/wk; men: <7, 7–14.9, 15–24.9, or ≥25 MET-h/wk), alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, or ≥7.5 g/d), pack-years of smoking (0, 1–15, 16–25, 26–45, or >45 pack-years), AHEI dietary score (women: <37.8, 37.8–43.5, 43.6–49.9, or >49.9; men: <35.2, 35.2–40.9, 41.0–47.6, or >47.6), multivitamin use (yes or no), regular aspirin/NSAID use (yes or no), fasting status (fasting or nonfasting), menopausal hormone therapy (women only: past use, current use, or no use), and menopausal status (women only: premenopause, postmenopause, or uncertain menopause).
4Multivariate linear regression models with adjustment for covariates included in model 2 and BMI at blood draw (continuous).
To examine the influence of achieved BMI level on trajectory-biomarker associations, we further adjusted for BMI at blood draw (model 3). In both sexes, differences in C-peptide and IGFBP-1 concentrations were attenuated to null for participants with a medium–marked increase trajectory, whereas being lean and gaining a substantial amount of weight (i.e., the lean–marked increase group) was still associated with higher C-peptide (women only) and lower IGFBP-1 concentrations. Specifically, women with a lean–marked increase trajectory had significantly higher C-peptide (percentage of difference: 10%; 95% CI: 5%, 15%) and lower IGFBP-1 (–18%; 95% CI: –31%, –3%) concentrations compared with the reference group, whereas men with a lean–marked increase trajectory had significantly lower concentrations of IGFBP-1 (–21%; 95% CI: –35%, –6%).
The importance of current BMI for C-peptide and IGFBP-1 concentrations is also shown by the joint analysis (Supplemental Figure 2), in which biomarker concentrations differed substantially by categories of attained BMI within each trajectory group.
Discussion
We observed that participants with a heavy body shape trajectory had a more unfavorable biomarker profile in adult life, as indicated by higher C-peptide and lower IGFBP-1 concentrations, than those who were lean across the life course. The trajectory-biomarker associations were largely driven by attained BMI, and to a lesser extent, by weight gain in early-middle adulthood.
Several studies have examined associations between indicators of adiposity at select time points and biomarker concentrations of the insulin-IGF pathway. However, few have examined associations of adiposity across the life span in relation to concentrations of these biomarkers.
We found that participants experiencing a marked increase in body fatness across the life span had substantially higher C-peptide concentrations in adult life than those who were lean throughout life. The relations were largely driven by attained BMI level, although early-middle adult weight gain seemed to have an additional effect. These results are not surprising, given the well-known associations of obesity and middle-adulthood weight gain with insulin resistance (12). At the molecular level, excess energy and progressive adipocyte enlargement may result in oversaturation of adipocytes, leading to their rupture, invasion of macrophages, and dysregulated adipokine secretion. These pathophysiologic changes may promote a diabetogenic environment (5) characterized by reduced tissue responsiveness to insulin and a compensatory increase in circulating insulin concentrations.
IGFBP-1 has been shown to be negatively associated with adult adiposity in several studies (27–29). In line with these observations, we observed that participants who gained a substantial amount of weight had markedly lower IGFBP-1 concentrations than those who stayed lean. Similar to C-peptide, adult IGFBP-1 concentrations were mainly determined by the ultimate BMI, with middle-adult weight gain exerting an additional effect. Similar to our observations, Rowlands et al. (30) observed a strong negative association in 1106 healthy men between attained BMI, change in body shape, and adulthood concentrations of IGFBP-2, which like IGFBP-1, is involved in modulation of the acute bioavailability of IGF-I (9).
Several observational studies have indicated an inverted U-shaped relation between adult adiposity and IGF-I concentrations, with the highest concentrations observed in participants with a BMI in the upper range of normal to moderate overweight (BMI: 24–27) (27, 28, 30, 31). Consistent with these data, we found that compared with the lean-stable group, participants who experienced a modest weight gain had a higher IGF-I concentration, whereas those with the heaviest body shape had a lower IGF-I concentration. It has been proposed that the inverted U-shaped relation is driven by obesity-associated abnormalities in insulin and growth hormone (GH) signaling (31). In catabolic states, low circulating insulin concentrations are accompanied by a reduction in GH receptor concentrations and resistance to GH-dependent stimulation of IGF-I synthesis. This leads to a reduction in the circulating concentrations of IGF-I, which has been observed in both normal-weight participants undergoing fasting (32) and anorectic patients (33). With increasing body weight and insulin concentrations, the liver becomes sensitized to the stimulatory effects of GH on IGF-I synthesis. In chronic hyperinsulinemia, increased IGF-I synthesis is accompanied by downregulation of the hepatic production of IGFBP-1 and IGFBP-2, resulting in increased circulating concentrations of free IGF-I. This induces a negative-feedback loop, resulting in reduced GH secretion and hence lower IGF-I concentrations. It is suggested that this negative-feedback mechanism plays a dominant role as adiposity concentrations continue to increase (31).
Studies examining associations between adult adiposity and IGFBP-3 concentrations have yielded inconsistent results. Some reported a weak positive association (24, 27, 28, 30, 31), whereas others reported no relation (29, 34). Our results indicate a modest positive association between body fatness and IGFBP-3 concentrations, with slightly higher concentrations in lean participants experiencing a moderate or marked weight gain than in participants who stayed lean. Our findings are in line with the report of Rowlands et al. (30) that showed a positive association between attained BMI, change in body shape, and adult IGFBP-3 concentrations.
In line with previous studies (30, 34), our results do not support an association between adiposity and the IGF-I–to–IGFBP-3 molar ratio. Although widely used as an indicator of IGF-I bioavailability, the validity of the IGF-I–to–IGFBP-3 ratio has been questioned (9).
Given the important role of the insulin-IGF system in chronic disease development (8, 35–38), research into modifiable risk factors of this system, such as adiposity, is of great value from a public health perspective. Our results support the importance of weight management across the life span for the prevention of metabolic complications and subsequent risk of chronic diseases. Because preventing weight gain may be more feasible than promoting and maintaining weight loss (39), clinicians and public health recommendations should focus on the prevention of weight gain during early to middle adulthood. However, this message does not contradict the recommendation for weight loss in individuals who are already overweight or obese.
Major strengths of this study include its large sample size and availability of detailed lifestyle data that allowed for rigorous control of confounding. Furthermore, we used a trajectory approach to incorporate data on body fatness at multiple time points across the life span and to directly compare biomarker concentrations between clusters of individuals with distinct adiposity trajectories. This approach respects the continuity of body growth and produces intuitive results about how lifetime body fatness may influence metabolic biomarkers. This represents a substantial advantage over previous studies that used data on body fatness at a few select time points only and assessed early- and late-life adiposity separately.
Our study also has some limitations. First, participants included in the current study represent a relatively small subset of the 2 cohorts (8% for both sexes). Nevertheless, basic characteristics of the included participants are relatively similar to those not included (Supplemental Table 6). Second, like other life-course epidemiologic studies, confounding patterns are complex and represent a challenge. For example, certain lifestyle factors, such as physical activity, can be both confounders and mediators. Thus, although adjusting for them may have minimized confounding, there may be overadjustment, resulting in underestimation of the trajectory-biomarker associations. Third, the self-reported somatotypes may be subject to measurement error. However, given the prospective design, any trajectory misclassification due to measurement error would likely be nondifferential with regard to biomarker concentrations, and hence result in underestimation of the true associations. Fourth, the identified trajectories may not accurately reflect each individual's course of body fatness. However, mean posterior probability and OCC were high in all groups, indicating good performance of our trajectory model. Finally, biomarkers were assessed at 1 time point only. Although these biomarkers have been shown to be relatively stable over shorter time periods (40, 41), their concentrations may change quite substantially over the life course, highlighting the need for repeated biomarker assessments over longer time periods. Also, it should be noted that biomarkers were assessed at an age when their concentrations are in the midst of an age-related decline (42). Whether trajectories of adiposity influence the rate of metabolic aging should be investigated further.
In conclusion, adiposity across the life span was associated with a more unfavorable biomarker profile in adult life, indicated by increased C-peptide and reduced IGFBP-1 concentrations. The trajectory-biomarker associations were largely driven by ultimate BMI and, to a lesser extent, weight gain in early-middle adulthood. Our findings support the importance of weight management across the life span for metabolic health and prevention of chronic diseases.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ASK and MS: designed the research, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, and wrote the manuscript; ATC, WCW, and ELG: provided essential materials (i.e., access to databases); ASK and DH: analyzed the data; MS: was responsible for study supervision and is the guarantor; and all authors: critically revised the manuscript for important intellectual content and read and approved the final manuscript. The authors had no conflicts of interest to disclose.
Notes
Supported by the NIH (K99CA215314, UM1 CA186107, P01 CA87969, R01 CA49449, R01 HL034594, R01 HL088521, UM1 CA167552, R01 HL35464; to MS, ELG, WCW, and ATC), the American Cancer Society (a Mentored Research Scholar Grant in Applied and Clinical Research, MRSG-17-220-01-NEC; to MS), and the Henning and Johan Throne-Holst foundation (to ASK). The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Supplemental Tables 1–6 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHEI, Alternative Healthy Eating Index; GH, growth hormone; HPFS, Health Professionals Follow-Up Study; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor–binding protein; NHS, Nurses’ Health Study; OCC, odds of correct classification.
REFERENCES
- 1. WHO. Obesity and overweight. [cited 2018 Feb 9]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/. [Google Scholar]
- 2. Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, Willett WC, Hu FB. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 2017;318(3):255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song M, Willett WC, Hu FB, Spiegelman D, Must A, Wu K, Chan AT, Giovannucci EL. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer 2016;138(10):2383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song M, Hu FB, Wu K, Must A, Chan AT, Willett WC, Giovannucci EL. Trajectory of body shape in early and middle life and all cause and cause specific mortality: results from two prospective US cohort studies. BMJ 2016;353:i2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, Martinez JA. Obesity. Nat Rev Dis Primers 2017;3:17034. [DOI] [PubMed] [Google Scholar]
- 6. Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med 2017;23(7):804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen DH, LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr Relat Cancer 2012;19(5):F27–45. [DOI] [PubMed] [Google Scholar]
- 8. Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol 2011;7(1):11–24. [DOI] [PubMed] [Google Scholar]
- 9. Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer 2014;14(5):329–41. [DOI] [PubMed] [Google Scholar]
- 10. Lee JM, Pilli S, Gebremariam A, Keirns CC, Davis MM, Vijan S, Freed GL, Herman WH, Gurney JG. Getting heavier, younger: trajectories of obesity over the life course. Int J Obes (Lond) 2010;34(4):614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr 2002;76(3):653–8. [DOI] [PubMed] [Google Scholar]
- 12. Hu F. Obesity epidemiology. Oxford (United Kingdom): Oxford University Press; 2008. [Google Scholar]
- 13. Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338(8765):464–8. [DOI] [PubMed] [Google Scholar]
- 14. Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health (Larchmt) 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 15. Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, Longcope C, Speizer FE. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst 1995;87(17):1297–302. [DOI] [PubMed] [Google Scholar]
- 16. Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst 2005;97(22):1688–94. [DOI] [PubMed] [Google Scholar]
- 17. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1(6):466–73. [DOI] [PubMed] [Google Scholar]
- 18. Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19(8):570–2. [PubMed] [Google Scholar]
- 19. Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Research publications—Res Publ Assoc Res Nerv Ment Dis 1983;60:115–20. [PubMed] [Google Scholar]
- 20. Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol 1993;138(1):56–64. [DOI] [PubMed] [Google Scholar]
- 21. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76(6):1261–71. [DOI] [PubMed] [Google Scholar]
- 23. Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol 2008;167(6):653–66. [DOI] [PubMed] [Google Scholar]
- 24. Holmes MD, Pollak MN, Hankinson SE. Lifestyle correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomark Prev 2002;11(9):862–7. [PubMed] [Google Scholar]
- 25. Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics 1983;25(2):165–72. [Google Scholar]
- 26. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010;6:109–38. [DOI] [PubMed] [Google Scholar]
- 27. Voskuil DW, Bueno de Mesquita HB, Kaaks R, van Noord PA, Rinaldi S, Riboli E, Grobbee DE, Peeters PH. Determinants of circulating insulin-like growth factor (IGF)-I and IGF binding proteins 1–3 in premenopausal women: physical activity and anthropometry (Netherlands). Cancer Causes Control 2001;12(10):951–8. [DOI] [PubMed] [Google Scholar]
- 28. Allen NE, Appleby PN, Kaaks R, Rinaldi S, Davey GK, Key TJ. Lifestyle determinants of serum insulin-like growth-factor-I (IGF-I), C-peptide and hormone binding protein levels in British women. Cancer Causes Control 2003;14(1):65–74. [DOI] [PubMed] [Google Scholar]
- 29. Schernhammer ES, Tworoger SS, Eliassen AH, Missmer SA, Holly JM, Pollak MN, Hankinson SE. Body shape throughout life and correlations with IGFs and GH. Endocr Relat Cancer 2007;14(3):721–32. [DOI] [PubMed] [Google Scholar]
- 30. Rowlands MA, Holly JM, Gunnell D, Gilbert R, Donovan J, Lane JA, Marsden G, Collin SM, Hamdy F, Neal DE. et al.. The relation between adiposity throughout the life course and variation in IGFs and IGFBPs: evidence from the ProtecT (Prostate testing for cancer and Treatment) study. Cancer Causes Control 2010;21(11):1829–42. [DOI] [PubMed] [Google Scholar]
- 31. Gram IT, Norat T, Rinaldi S, Dossus L, Lukanova A, Tehard B, Clavel-Chapelon F, van Gils CH, van Noord PA, Peeters PH. et al.. Body mass index, waist circumference and waist-hip ratio and serum levels of IGF-I and IGFBP-3 in European women. Int J Obes (Lond) 2006;30(11):1623–31. [DOI] [PubMed] [Google Scholar]
- 32. Clemmons DR, Underwood LE. Nutritional regulation of IGF-I and IGF binding proteins. Annu Rev Nutr 1991;11:393–412. [DOI] [PubMed] [Google Scholar]
- 33. Argente J, Caballo N, Barrios V, Munoz MT, Pozo J, Chowen JA, Morande G, Hernandez M. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in patients with anorexia nervosa: effect of short- and long-term weight recuperation. J Clin Endocrinol Metab 1997;82(7):2084–92. [DOI] [PubMed] [Google Scholar]
- 34. Schoen RE, Schragin J, Weissfeld JL, Thaete FL, Evans RW, Rosen CJ, Kuller LH. Lack of association between adipose tissue distribution and IGF-1 and IGFBP-3 in men and women. Cancer Epidemiol Biomark Prev 2002;11(6):581–6. [PubMed] [Google Scholar]
- 35. Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, Andreotti F. Insulin-like growth factor-1 as a vascular protective factor. Circulation 2004;110(15):2260–5. [DOI] [PubMed] [Google Scholar]
- 36. Ezzat VA, Duncan ER, Wheatcroft SB, Kearney MT. The role of IGF-I and its binding proteins in the development of type 2 diabetes and cardiovascular disease. Diabetes Obesity Metab 2008;10(3):198–211. [DOI] [PubMed] [Google Scholar]
- 37. Kotronen A, Lewitt M, Hall K, Brismar K, Yki-Jarvinen H. Insulin-like growth factor binding protein 1 as a novel specific marker of hepatic insulin sensitivity. J Clin Endocrinol Metab 2008;93(12):4867–72. [DOI] [PubMed] [Google Scholar]
- 38. Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001;24(4):683–9. [DOI] [PubMed] [Google Scholar]
- 39. Kassirer JP, Angell M. Losing weight—an ill-fated New Year's resolution. N Engl J Med 1998;338(1):52–4. [DOI] [PubMed] [Google Scholar]
- 40. Missmer SA, Spiegelman D, Bertone-Johnson ER, Barbieri RL, Pollak MN, Hankinson SE. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomark Prev 2006;15(5):972–8. [DOI] [PubMed] [Google Scholar]
- 41. Muti P, Trevisan M, Micheli A, Krogh V, Bolelli G, Sciajno R, Berrino F. Reliability of serum hormones in premenopausal and postmenopausal women over a one-year period. Cancer Epidemiol Biomark Prev 1996;5(11):917–22. [PubMed] [Google Scholar]
- 42. Bann D, Holly JMP, Lashen H, Hardy R, Adams J, Kuh D, Ong KK, Ben‐Shlomo Y. Changes in insulin‐like growth factor‐I and ‐II associated with fat but not lean mass in early old age. Obesity (Silver Spring) 2015;23(3):692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.