Figure 1.
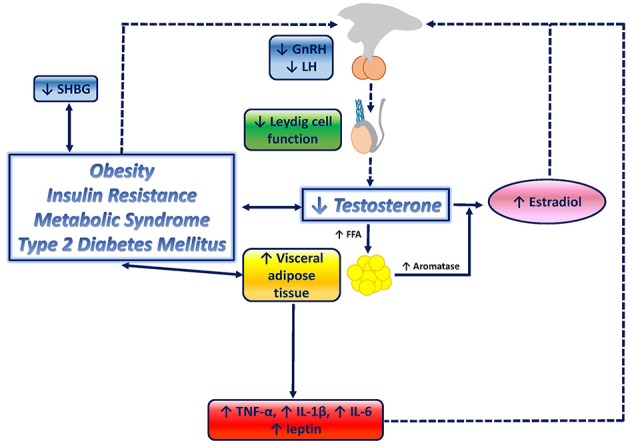
Graphical overview of the relationship between testosterone deficiency and metabolic disorders. Testosterone deficiency determines an increase in lipoprotein lipase activity, resulting in increased fatty acids (FFA) uptake and triglyceride levels in adipocytes, which ultimately stimulate adipocyte proliferation and visceral adipose tissue (VAT) increase, and therefore, contribute to the onset of visceral obesity. VAT accumulation triggers several downstream mechanisms which contribute to further impair the metabolic profile, and, potentially, enhances testosterone deficiency by closing the loop: increased aromatase activity results in increased conversion of testosterone to estradiol; inflammatory mediators, known to be increased in metabolic disorders, including tumor necrosis factor-a (TNF-a), interleukin-6 (IL-6), and interleukin-1b (IL-1b), have been shown to suppress hypothalamic gonadotropin releasing hormone (GnRH), and consequently, pituitary luteinizing hormone (LH) and testosterone secretion; increased leptin levels exert direct inhibitory effects on Leydig cells; leptin resistance at the hypothalamic-pituitary level, mediated by reduced LH release, boosts testosterone suppression; insulin resistance (IR) associated with obesity, metabolic syndrome and type-2 diabetes mellitus has been suggested to reduce gonadotropin secretion, therefore contributing to testosterone deficiency, and to reduce sex hormone binding globulin (SHBG).
