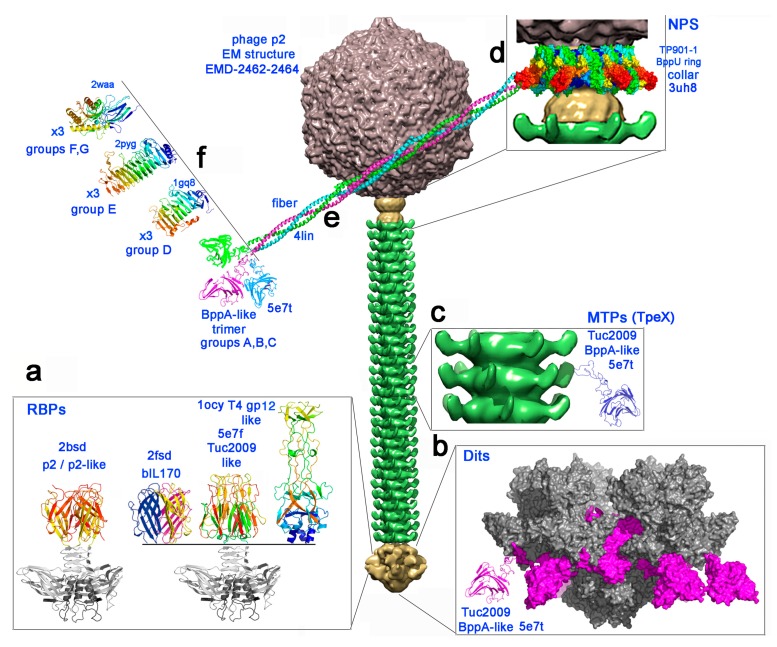
Figure 1.
The carbohydrate binding modules of 936 group phages represented on the electron microscopy structure of phage p2 [32]. From top to bottom, the capsid (grey), the connector (yellow), the tail (green) and the baseplate (yellow). (a) The four types of receptor binding protein head domains [10] found in the 936 family. The constant shoulders and neck domain is depicted in grey, and the head domains are rainbow colored. (b) A structural model (molecular surface) of the previously described [9] BppA insertion (violet) within the evolved Dit arm from a 936 phage baseplate (grey) [8]. (c) The TpeX BppA-containing extension is represented in ribbon mode in blue, inserted after the protruding Major Tail Protein (MTP) adhesin domain. (d–f) The model of the neck passage proteins with the collar surrounding the connector (d, molecular surface), the triple helix bundle of the six fibers (e, ribbon mode), terminated by one of the four possible trimeric carbohydrate binding modules (CBM) domains (f, ribbon mode).