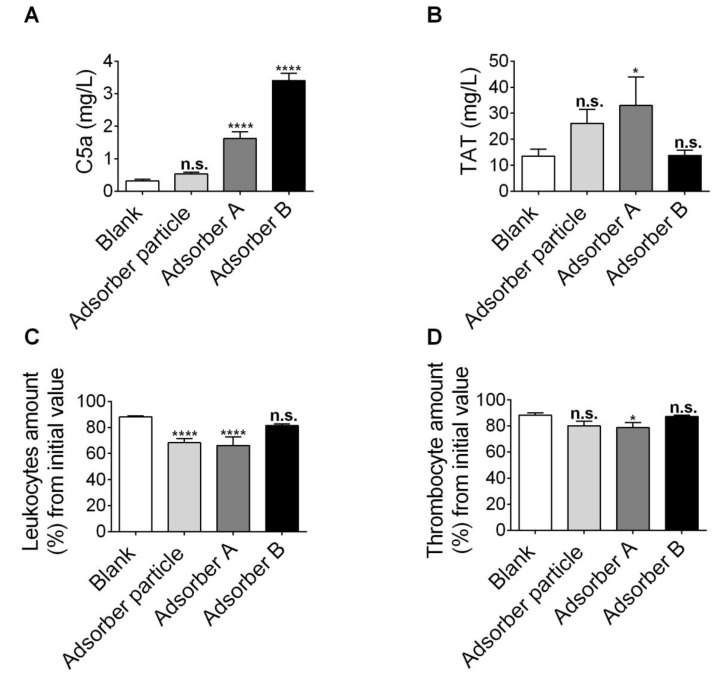
Figure 4.
Hemocompatibility assessment of the newly developed adsorber particle. Hemocompatibility was assessed after flowing human blood for 180 min through a cartridge filled with the adsorber particles at a flow rate of 12.6 mL/min. One approach without particles was used as a blank. (A,B) Quantification of complement component 5a (C5a) (A) and the thrombin antithrombin-complex III (TAT) (B) in blood after incubation with newly developed whole-blood adsorber, adsorber A or adsorber B particles, or without particles (blank), as indicated. (C,D) Relative leukocyte count (C) and thrombocyte count (D) in blood after incubation with the newly developed whole-blood adsorber, non-primed adsorber A or adsorber B particles, or without particles (blank), as indicated, and displayed in % from the initial value in blood. (A–D) Data are given as mean values ± S.E.M; n = 15 for blank, n = 12 for the newly developed adsorber particle, n = 9 for adsorber A, and n = 5 for adsorber B. * p < 0.05, **** p < 0.0001, n.s. = not significant. One-way ANOVA with Dunnet’s multiple comparison test were all compared to the blank.