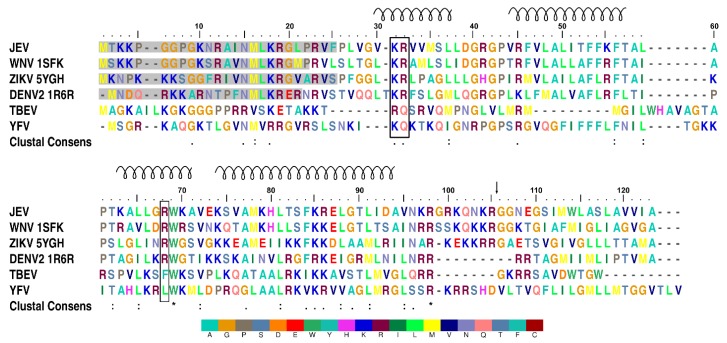
Figure 1.
Multiple sequence alignment of flavivirus capsid proteins. Positively charged residues, arginine (R) and lysine (K), accumulate at the N- and C-termini. Lysine 31, arginine 32, and arginine 68 are highlighted with a black box. The spiral above the sequence indicates the α-helical secondary structure and numbering, which is based on the JEV capsid protein. Residues that are not visible in protein structures are shaded in grey. The black arrow marks the natural NS3 protease cleavage site. An asterisk indicates a fully conserved residue. A colon indicates conservation between groups of strongly similar properties. A period indicates conservation between groups of weakly similar properties. The alignment was produced with MUSCLE [40]. Color is given to each residue with the legend below.