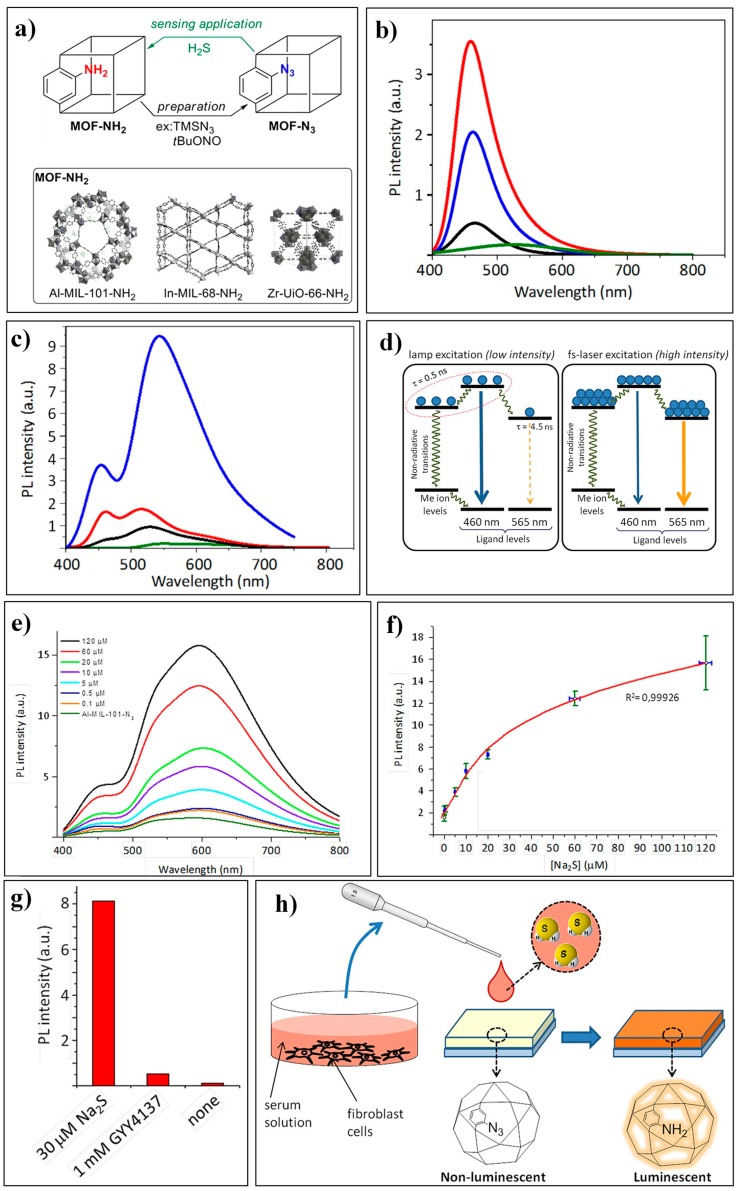
Figure 3.
(a) Azido-MOFs for H2S sensing. (b,c) Emission spectra of dry MOF samples, Al-MIL-101-NH2 (blue), In-MIL-68-NH2 (red), Zr-UiO-66-NH2 (black), Al-MIL-101-N3 (green) under UV-lamp excitation (b) or fs-pulse laser excitation (c) at 343 nm. (d) Proposed schematic mechanisms of charges recombination underpinning the observed switch in emission channels. (e) Emission spectra of Al-MIL-101-N3 in the presence of different concentrations of sodium sulfide (excited at 343 nm with a fs-pulse laser). (f) Reversed cubic fit of sodium sulfide concentration vs. measured intensity of the 565 nm band in emission spectra. (g) Detection of H2S released from GYY4137. (h) Exposure of Al-MIL-101-N3 to a sample of culture medium for cells with endogenously produced H2S. Reproduced with permission from [79]. Copyright Wiley-VCH, 2016.