Figure 7. Schematic model of RAKEC between Tiam1 and CaMKII for persistence of sLTP.
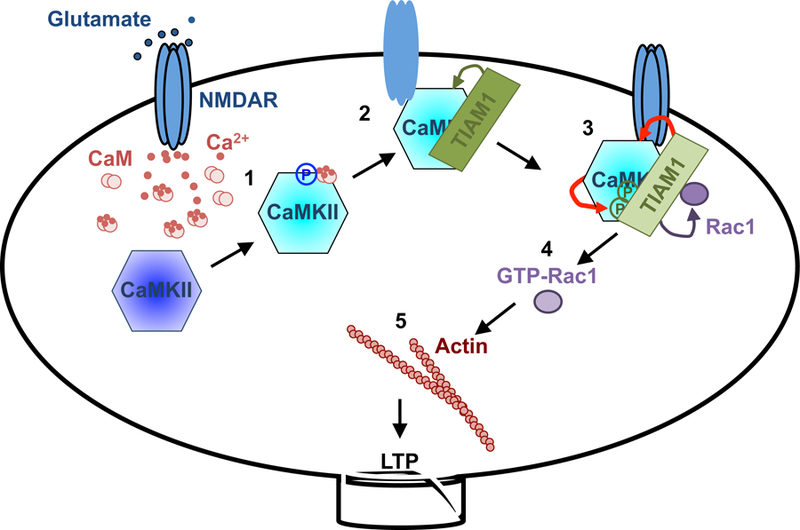
1. Ca2+ influx through NMDAR increases Ca2+/CaM binding to CaMKII, which activates the kinase and results in T286 autophosphorylation. Phosphorylated T286 disinhibits the kinase from autoinhibition.
2. Ca2+/CaM-activated CaMKII binds to Tiam1 and NR2B subunit of NMDAR. Pseudoautoinhibitory domain on Tiam1 dislodges the CaMKII autoinhibitory domain and prevents it from inhibiting the kinase.
3. In the protein complex with Tiam1, CaMKII phosphorylates and activates Tiam1 (RAKEC). This mechanism prolongs the kinase activity, which in turn maintains the phosphorylated status of Tiam1 and, therefore, its RacGEF activity.
4. Phosphorylated Tiam1 activates Rac1.
5. Rac1 promotes actin remodeling to maintain spine morphology.
Blue: inactive CaMKII, cyan: activated CaMKII, Red arrows: reciprocal activation.
