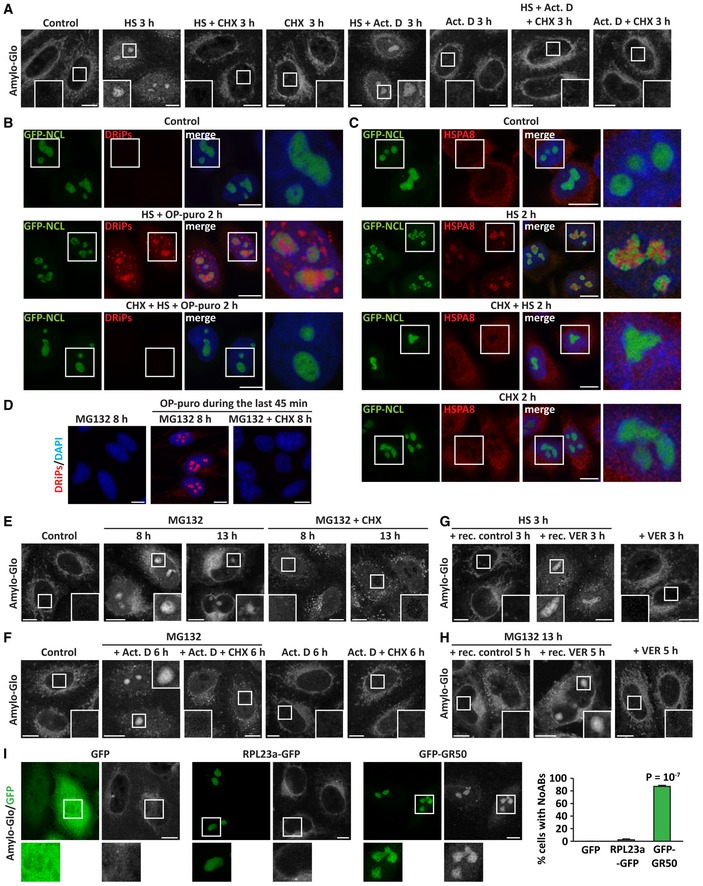
HeLa cells were left untreated or treated with heat shock (HS) at 42°C for 3 h, alone or with cycloheximide (CHX; 50 μg/ml), or actinomycin D (Act. D; 3 μM) followed by staining with the dye Amylo‐glo.
DRiP labeling in GFP‐NCL HeLa Kyoto cells that were either left untreated or treated with HS at 42°C and OP‐puro (25 μM) for 2 h. Where indicated, cells were co‐treated with CHX (50 μg/ml).
HSPA8 subcellular distribution and recruitment inside nucleoli following exposure to HS at 42°C for 2 h, alone or combined with CHX (50 μg/ml), in GFP‐NCL HeLa Kyoto cells.
DRiP labeling in HeLa cells treated with MG132 (10 μM) alone or with CHX (50 μg/ml) for 8 h; where indicated, OP‐puro (25 μM) was added during the last 45 min, prior to cell fixation.
Amylo‐glo staining of HeLa cells left untreated or exposed to MG132 (10 μM) alone or combined with CHX (50 μg/ml) for 8 or 13 h.
Amylo‐glo staining of HeLa cells left untreated or subjected to transcriptional stress (MG132 10 μM and Act. D 4 μM) for 6 h. Where indicated, translation was concomitantly inhibited with CHX (50 μg/ml). Amylo‐Glo staining is also shown in HeLa cells treated with Act. D alone or with CHX for 6 h, as control.
Amylo‐glo staining of HeLa cells exposed to HS at 42°C for 3 h and let to recover for 3 h in drug‐free medium (+ rec. control) or with VER (40 μM; + rec. VER). Where indicated, cells were treated with VER alone (VER 3 h).
Amylo‐glo staining of HeLa cells treated with MG132 (10 μM) for 13 h and let to recover for 5 h in drug‐free medium (+ rec. control) or with VER (40 μM; + rec. VER). Where indicated, cells were treated with VER alone (VER 5 h).
HeLa cells were transfected with vectors encoding for GFP, GFP‐GR50, or RPL23a‐GFP. 24 h post‐transfection, cells were fixed and stained with Amylo‐glo. The percentage of transfected cells with Amylo‐Glo‐positive nucleoli is shown. Number of cells counted/condition: GFP, 412; RPL23a‐GFP, 482; GFP‐GR50, 359, in three independent experiments; statistical significance via one‐way ANOVA; P < 10−7, ± s.e.m.
Data information: (A–I): Scale bars: 10 μm.