Abstract
National data on dengue notifications do not capture all dengue infections and do not reflect the true intensity of disease transmission. To assess the true dengue infection rate and disease control efforts in Singapore, we conducted age-stratified serosurveys among residents after a 2013 outbreak that was the largest dengue outbreak on record. The age-weighted prevalence of dengue immunoglobulin G among residents was 49.8% (95% confidence interval: 48.4, 51.1) in 2013 and 48.6% (95% confidence interval: 47.0, 50.0) in 2017; prevalence increased with age. Combining these data with those from previous serosurveys, the year-on-year estimates of the dengue force of infection from 1930 to 2017 revealed a significant decrease from the late 1960s to the mid-1990s, after which the force of infection remained stable at approximately 10 per 1,000 persons per year. The reproduction number (R0) had also declined since the 1960s. The reduction in dengue transmission may be attributed to the sustained national vector program and partly to a change in the age structure of the population. The improved estimated ratio of notified cases to true infections, from 1:14 in 2005–2009 to 1:6 in 2014–2017, signifies that the national notification system, which relies on diagnosed cases, has improved over time. The data also suggest that the magnitudes of dengue epidemics cannot be fairly compared across calendar years and that the current disease control program remains applicable.
Keywords: basic reproduction number, Bayesian model, dengue, force of infection, infectious disease, seroprevalence, vector control
Dengue viral infection is a major threat to global health. Numbers of reported cases across the Americas, Southeast Asia, and the western Pacific exceeded 1.2 million in 2008 and 3 million in 2013 (1, 2). Dengue notifications have been likened to the tip of an iceberg, however, as there are many more infections than reported, because of asymptomatic infections and infections that are misdiagnosed or for which medical attention is not sought (3).
Several prospective cohort studies conducted in Thailand and Nicaragua showed that numbers of infections were 8- to 20-fold higher than national numbers of reported cases (4, 5). Inapparent infection obscures estimates of the true number of cases of this reemerging disease (6–9). Recently, modeling conducted to map the global distribution of dengue risk estimated 390 million dengue infections per year, of which only 96 million are symptomatic (10). This estimate is at least thrice the figure provided by the World Health Organization (11).
Dengue is endemic to the equatorial city-state of Singapore, where multiple serotypes cocirculate and dengue cases are reported throughout the year. Since the resurgence of dengue in the 1980s, Singapore has experienced epidemics of increasing magnitudes over the past 3 decades (12–17). In 2013, Singapore saw the highest number of reported cases recorded, with incidence at 404.9 cases per 100,000 population, corresponding to more than 22,000 notified cases (18). Although all 4 dengue virus serotypes cocirculate in Singapore, dengue virus type 1 (DENV-1) (2004–2006, 2013–2014) and dengue virus type 2 (DENV-2) (2007–2012, 2015–2017) have been the predominant serotypes (17–20).
Despite the apparent increase in infections, the prevalence of anti–dengue immunoglobulin G (IgG) antibodies among adult residents of Singapore has fallen over the last few decades (21). The extent to which this ostensible contradiction can be attributed to changes in diagnostic procedures and notification rates and what this implies for dengue epidemiology in Singapore are not clear.
To address this uncertainty, we conducted 2 cross-sectional serosurveys after the 2013 dengue epidemic, to compare them with previous surveys. In contrast to longitudinal cohort studies, repeated cross-sectional serosurveys can be more cost-effective in settings like Singapore, where the force of infection (FOI)—that is, the rate at which susceptible individuals are infected (22)—is known to be low (23). Using Bayesian computational methods, we analyzed data from these serosurveys together with past serological data to estimate long-term temporal changes in the FOI, the basic reproduction number , and numbers of infections averted.
METHODS
Seroprevalence study
Sample selection
Residual serum samples were collected from nonremunerated blood donors by the Blood Services Group of the Singapore Health Sciences Authority from December 2013 to February 2014 and from June to August 2017. An initial sample size of 4,160 was determined for the study, based on an estimated seroprevalence of 50.8% (21) at a confidence level of 99% and precision of 2%. Samples were selected according to the age and sex distribution of the adult population in the years 2013 and 2017, as previously described (21). Population data were obtained from the Singapore Department of Statistics.
Ethics statement
The National Healthcare Group Domain Specific Review Board and the Bioethics Review Committee of the National Environment Agency/Environmental Health Institute approved the 2013 and 2017 studies, respectively. Written informed consent to test the serum samples for infectious diseases was obtained from the blood donors as part of the Donor Health Assessment Questionnaire. In addition, parental consent was obtained for donors below 18 years of age. Donor identifiers were removed, and only the age, sex, residency status, and residential postal code of each participant were retained.
Serological analysis
The presence of dengue IgG in the serum samples was determined by enzyme-linked immunosorbent assay (ELISA) using the Panbio Dengue IgG Indirect ELISA (Alere Inc., Waltham, Massachusetts), according to the manufacturer’s instructions.
Data analysis
Only data on Singapore residents (citizens and permanent residents of Singapore; n = 3,813 in 2013 and n = 4,002 in 2017) were analyzed. Dengue IgG prevalence was weighted to adjust for age as previously described (21). Fisher’s exact test was used to assess the significance of differences in seropositivity between groups. Prevalence ratios and 95% Wald confidence intervals were computed for each group as compared with the referent. A 2-sample z test for equality of proportions with continuity correction was used to determine whether differences in dengue IgG prevalence between age groups in 2009 and 2013 were statistically significant. Statistical significance was defined as P < 0.05.
Bayesian model for FOI
Model development
Age-specific dengue seroprevalence data independently collected in 2004 and 2009 (including the pediatric cohort) were integrated with data from the 2013 and 2017 serological surveys to estimate the historical dengue FOI (see Table 1 and Web Appendix 1, available at https://academic.oup.com/aje) (21, 24, 25). The IgG ELISA test commonly used in seroprevalence studies is unable to distinguish between dengue serotypes or to determine the number of different dengue infections an individual has contracted. In addition, the test may produce false-negative results due to degradation of sensitivity over time, especially for patients whose most recent infection occurred decades before the test was conducted. For this analysis, we estimated an age-independent, discrete-time FOI (denoted by ), defined as the rate at which a dengue-naive (seronegative) individual acquired primary infection with any serotype during year ; we assumed that the FOI could vary yearly but was common to all age groups during each calendar year (23). We also assumed that individuals lost seropositivity as detected by the IgG ELISA test at a constant rate α, the prior distribution of which was based on Imai et al.’s (26) estimate. Hence, given serosurvey participants aged a years in year y, the number of persons having a positive IgG ELISA test result was assumed to follow a binomial distribution: , where
Table 1.
Summary of 5 Dengue Serosurveys in Singapore, 2004–2017
| Survey | Agency or Institution | Age Range, years | Sample Size (n) | Survey Period | ELISA Testa | Source (First Author, Year (Reference No.)) |
|---|---|---|---|---|---|---|
| National Health Surveyb | MOH | 18–74 | 4,152 | September–December 2004 | Panbio Dengue IgG Indirect ELISAc | Yew, 2009 (24) |
| National Paediatric Seroprevalence Surveyb | MOH | 2–15 | 984 | August 2008–July 2010 | EUROIMMUN Anti-Dengue Virus ELISA (IgG)d | Ang, 2015 (25) |
| 2009 seroprevalence surveyb | NEA/EHI | 16–60 | 3,627 | December 2009–February 2010 | Panbio Dengue IgG Indirect ELISA | Low, 2015 (21) |
| 2013 seroprevalence survey | NEA/EHI | 16–71 | 3,813 | December 2013–February 2014 | Panbio Dengue IgG Indirect ELISA | Present study |
| 2017 seroprevalence survey | NEA/EHI | 16–74 | 4,002 | June–August 2017 | Panbio Dengue IgG Indirect ELISA | Present study |
Abbreviations: EHI, Environmental Health Institute; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; MOH, Ministry of Health; NEA, National Environment Agency.
a The sensitivity (Panbio: 97.9%; EUROIMMUN: 100%) and specificity (Panbio: 100%; EUROIMMUN: 100%) of the Panbio and EUROIMMUN assays (as provided in the product inserts) were similar. Both assays were tested in a serum sample panel of patients with suspected dengue virus infection (n = 87), and the qualitative results of the two ELISAs were 99% in agreement (EUROIMMUN Anti-Dengue Virus ELISA (IgG) product information). This shows that the data from these studies may be used collectively for analysis. The total number of samples analyzed in these 5 studies was 16,578.
b A brief description of prior studies can be found in Web Appendix 1.
c Alere Inc., Waltham, Massachusetts.
d EUROIMMUN AG, Lübeck, Germany.
For computational convenience, we worked with , which has support on the real line. A first-order Gaussian random walk prior distribution was specified to impose smoothness in the estimates: , where and the precision parameter was assigned a weakly informative prior: . The posterior distribution for was sampled using the Markov chain Monte Carlo method over 170,000 iterations. We discarded the initial 20,000 iterations as burn-in data and subsequently saved every 30th iteration to obtain a posterior sample of size 5,000.
Evaluation of model
We derived posterior median values for the proportion of individuals having a positive IgG ELISA test result for every combination of age and serosurvey year. These were used as null values within binomial tests to highlight observations with a statistically significant deviation from the model (at a 5% significance level), and the proportion of statistically significant observations was then tabulated (Web Appendix 2). In addition, the agreement between the empirical and reconstructed dengue IgG seroprevalences for every combination of age and serosurvey year was visually inspected, where Clopper-Pearson confidence intervals were used to quantify uncertainty resulting from potentially small sample sizes for each individual age.
Reconstructing seroprevalence over time
Assuming the risk of dengue infection among seronegative persons to be homogenous across age groups in a given year, we derived the posterior distribution of seroprevalence as a function of age and time from the FOI estimates and used it to obtain posterior distributions of the age-weighted seroprevalence during the period 1960–2017 (Web Appendix 2).
Estimating R0
Under the assumptions listed in Web Appendix 2, we estimated by converting the overall seroprevalence to a serotype-level prevalence estimate as a function of age and time using the relationship
where the subscript indicates that the prevalence is for 1 serotype. Age-weighted estimates of the serotype-level prevalence for each year were derived and applied to the following relationship to estimate as a function of time, y:
Justification for this approach and a sensitivity analysis which assesses the robustness of the estimates with respect to the percentage of annual resident cases being imported from abroad are provided in Web Appendix 2.
Calculation of the ratio of infections to notified cases
Estimation of total number of primary infections
The number of primary infections among Singapore residents during each year y was estimated using the population structure data, mortality rates, and the FOI estimated seroprevalence, where we assumed that mortality and serostatus were independent for each age group:
In the equation above, and denote the total numbers of seropositive residents at the end of year y and year (y − 1), respectively, derived using the FOI estimates. , , and stand for the midyear total number of residents, mortality rate, and midyear seroprevalence estimate for persons of age a in year y.
Estimation of infection:notification ratio
To estimate the infection:notification ratio for the period (, ), we divided the estimated total number of dengue infections among Singapore residents by the number of reported cases obtained from the Communicable Diseases Division of the Ministry of Health:
Here, is the total number of reported cases from years to , and the total number of primary infections was multiplied by 2, taking into account the ratio of 1:1 for primary:subsequent dengue infections. This factor was based on a locally conducted study, the Early Dengue Infection and Outcome (EDEN) Study, which revealed almost equal numbers of symptomatic dengue patients with primary and subsequent infections (52% and 48%, respectively) (27); this echoes Sabin’s landmark studies of the early 20th century (28). We applied the equation above to obtain estimated infection:notification ratios for periods between serosurveys (2005–2009, 2010–2013, 2014–2017) and for periods of DENV-1 (2005–2006, 2013–2014) and DENV-2 (2007–2012, 2015–2017) predominance.
Estimation of the number of primary infections averted
To estimate the number of primary infections averted among Singapore residents in 2017 because of changes in the FOI, the posterior samples of age-specific seroprevalence in 2017 were obtained according to 4 hypothetical situations (FOI held fixed at the level of the 1960s, 1970s, 1980s, and 1990s averages). In each situation, the difference between the age-specific seroprevalence based on the original FOI estimate and that obtained using the “frozen” hypothetical FOI was multiplied by the number of residents of each age in the year 2017. The total number of infections averted was multiplied by 2, taking into account the 1:1 ratio for primary:subsequent dengue infections (27), with a sensitivity analysis considering alternative values for this ratio (2:1 and 1:2) to account for potential biases caused by using symptomatic cases to determine the ratio of primary infections to nonprimary infections.
All mathematical and statistical analyses were performed using R software (R Foundation for Statistical Computing, Vienna, Austria) (29).
RESULTS
2013 and 2017 dengue seroprevalence studies
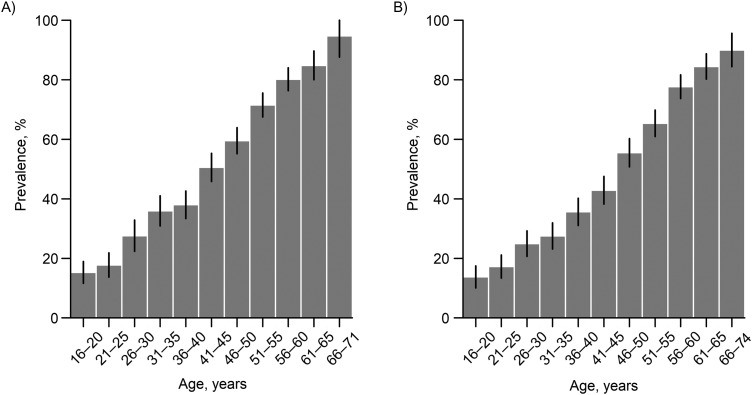
Among the persons sampled (n = 3,813 in 2013; n = 4,002 in 2017), dengue IgG prevalence declined from 49.1% (1,874/3,813; 95% confidence interval (CI): 47.6, 50.7) in 2013 to 45.7% (1,828/4,002; 95% CI: 44.1, 47.2) in 2017. The age-weighted prevalences in 2013 and 2017 were 49.8% (95% CI: 48.4, 51.1) and 48.6% (95% CI: 47.0, 50.0), respectively. The prevalence of dengue IgG antibodies increased with age (Figure 1), in agreement with previous serosurveys conducted in Singapore (21, 24, 30), from 15.3% (2013) and 13.8% (2017) among residents aged 16–20 years to 87.9% (2013) and 85% (2017) among those over age 60 years; the prevalence of having dengue IgG antibodies increased 1.08–1.55 times for every 5-year increase in age in 2013 and 1.14–1.85 times in 2017. Males were more likely to be seropositive than females (Web Tables 1 and 2). In the age group 16–60 years (which matches the 2009 serosurvey (21)), there was a significant decline (Fisher’s exact test: P < 0.05) in weighted IgG prevalence from 2009 (50.8%; 95% CI: 49.4, 52.3) to 2013 (44.8%; 95% CI: 43.3, 46.3) and 2017 (41.2%; 95% CI: 40.0, 42.7).
Figure 1.
Age-specific prevalence of dengue immunoglobulin G (IgG) in serological samples collected in Singapore in 2013 (A) and 2017 (B). The presence of dengue IgG in the serum samples was determined using the Panbio Dengue IgG Indirect ELISA (Alere Inc., Waltham, Massachusetts). Confidence intervals (black bars) were constructed using Wald's method. Participants aged 66 years or more were combined into one group because of the small sample size. ELISA, enzyme-linked immunosorbent assay.
Estimates of dengue FOI
The Bayesian estimates of the FOI were above 0.10 per annum until the 1960s, peaking at 0.178 (95% Bayesian credible interval (BCrI): 0.068, 0.511) in 1940 (Figure 2A). The estimated FOI gradually declined over the next 2 decades and remained at basal levels of around 0.009 in 1993 (95% BCrI: 0.006, 0.013) and 2017 (95% BCrI: 0.005, 0.015). Similar trends in FOI estimates were observed in models generated using individual data sets from 2004, 2009, 2013, and 2017 (Web Figure 1). The slight blips observed in recent years (Figure 2B) appeared to correspond to the large outbreaks experienced in 2005 and 2007. The rate of degradation of antibody detection was estimated to be 0.003 per year (2.5th and 97.5th percentiles of the posterior distribution: 0.002, 0.004), which is lower than the 2015 estimate made by Imai et al. (26).
Figure 2.
Estimated annual dengue force of infection (FOI) in Singapore during the periods 1931–2017 (A) and 1990–2015 (B). An overall declining trend in FOI estimates was observed from 1931 to 2017 (A); however, the FOI estimates increased slightly from 1999 to 2008 and then decreased (B). Solid lines represent the point estimates, and shaded regions represent the 95% Bayesian credible intervals. The model was developed using 2004, 2009, 2013, and 2017 serosurvey data.
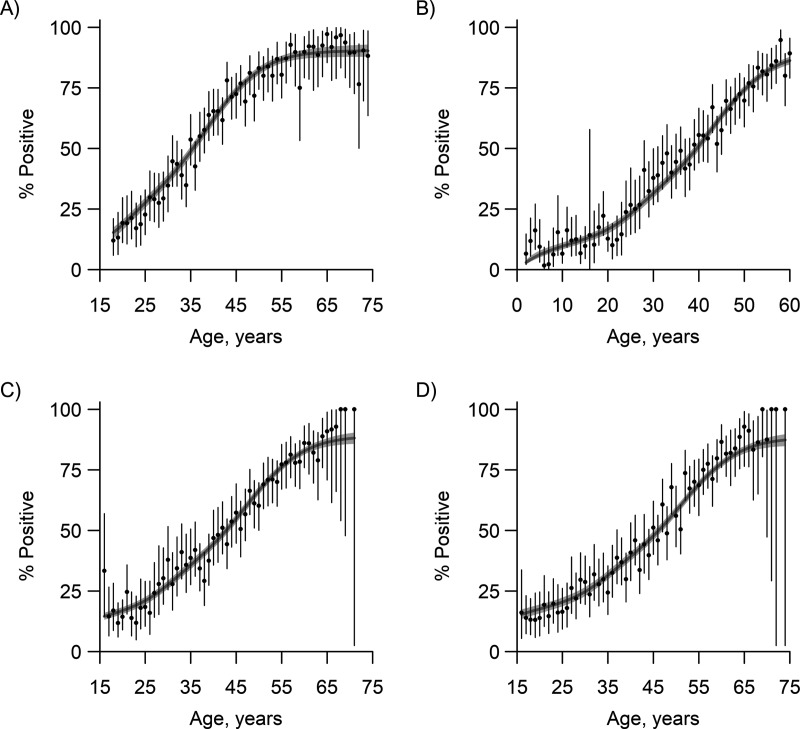
The observed and reconstructed age-stratified prevalence rates from the serosurveys are presented in Figure 3. The goodness of fit of the age-stratified seroprevalence estimates was found to be reasonable for the 2004, 2013, and 2017 data (Table 2). For the pediatric data from 2009, however, there was a notable discrepancy in the youngest age groups, which had empirical estimates for seroprevalence that were higher than the model predicted. Excluding children under 5 years of age from the 2009 cohort produced more adequate estimates, though there was a small cluster of outlying results in young adults. The extent to which these discrepancies reflect biases in recruitment or the impact of 2 large outbreaks (2005 and 2007) occurring prior to sampling in 2008–2010 is unclear.
Figure 3.
Comparison of empirical and reconstructed prevalences of dengue immunoglobulin G from independent serosurveys carried out in Singapore in 2004, 2009, 2013, and 2017. Empirical estimates are shown as black dots and 95% Clopper-Pearson confidence intervals by black bars. Solid gray lines represent the model estimates for 2004 (A), 2009 (B), 2013 (C), and 2017 (D), with the gray shaded regions representing 95% Bayesian credible intervals. Degradation of the sensitivity of the enzyme-linked immunosorbent assay over time was taken into account in the reconstructed estimates.
Table 2.
Goodness-of-Fit Tests of Reconstructed and Empirical Prevalence From Dengue Serosurveys in Singapore, 2004–2017
| Year | Age Range for Data Set, years | Age(s) With Significant (P < 0.05) Deviation From Model, years | % of Age Groups With Significant (P < 0.05) Deviation From Model |
|---|---|---|---|
| 2017 | 16–74 | 49 | 2 |
| 2013 | 16–71 | 16 | 2 |
| 2009 | 2–60 | 3, 4, 28, 58 | 7a |
| 2004 | 18–74 | 34, 49 | 4 |
a This figure became 4% after exclusion of children under 5 years of age.
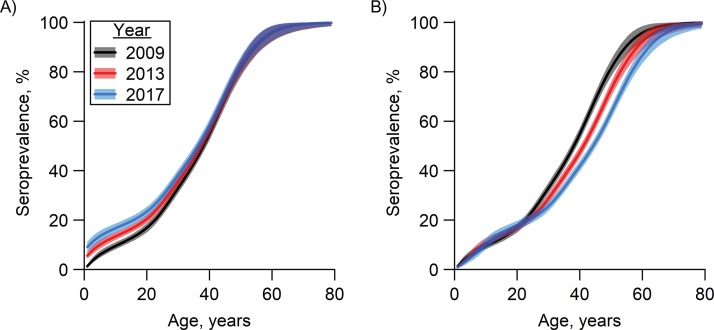
The observed and reconstructed prevalence rates, together with the reconstructed prevalence rates of 2009, 2013, and 2017, indicate that the prevalence rose marginally within a birth cohort over this time period (Figure 4A). However, comparing residents of the same age in 2009, 2013, or 2017, the estimated prevalence for persons over age 23 years was lower in 2013 than that for their counterparts in 2009, and similar in 2017 as compared with 2013 (Figure 4B). This indicated that despite the endemicity of symptomatic dengue incidence in the community, the population level of immunity within any adult age group showed a decrease from 2009 to 2017.
Figure 4.
Dengue seroprevalence by age and year among residents of Singapore, generated with Bayesian modeling, 2009, 2013, and 2017. Lines represent posterior median values for seroprevalence, and shaded areas represent 95% Bayesian credible intervals. A) Seroprevalence in the years 2009, 2013, and 2017 based on age in 2009. Within each birth cohort, only a marginal increase in seroprevalence is observable from 2009 to 2013 and from 2013 to 2017. The gap is most prominent in the youngest age group. B) Seroprevalence in the years 2009, 2013, and 2017 based on age in 2009, 2013, and 2017, respectively. Holding age fixed (in 2009, 2013, and 2017), the estimated prevalence for persons over age 23 years was lower in 2013 than in 2009 and lower in 2017 than in 2013.
Estimates of R0
Estimates of were above 1.4 per annum before the late 1990s, peaking at 2.02 (95% BCrI: 1.572, 2.656) in 1960. The estimates gradually declined over the next decade to 1.30 (95% BCrI: 1.264, 1.345) in 2013 and 1.28 (95% BCrI: 1.248, 1.319) in 2017 (Figure 5A). The reduced transmission rate indicated by estimates of corroborates the FOI estimates, the latter of which reflect a combination of infection risk and the changing age profile of the population. As compared with a previous study which showed varying levels of FOI and for Singapore (26), in the current study we used a more recent data set and a larger sample size across 4 time points to update historical FOI estimates, and we excluded studies that were not comparable with this national study (Web Table 3). Overall, our estimates were found to be robust with respect to the percentage of resident cases being imported from abroad, especially for the post-1990 estimates (Web Figure 2).
Figure 5.
Estimated annual basic reproduction number (R0) for dengue (A) and reconstructed annual dengue seroprevalence (B) in Singapore, 1960–2020. Solid lines represent the point estimates, and shaded regions represent 95% Bayesian credible intervals.
Seroprevalence against the backdrop of a change in age structure
A change in the age structure of the population due to a reduced birth rate and longer life expectancy is known to lower the disease transmission rate through a decrease in the proportion of the population that is susceptible. Because Singapore has seen a progressively aging population in recent decades, we explored whether the lower transmission rate could be attributed to such a dilution effect. We analyzed and reconstructed seroprevalence trends using age-stratified seroprevalence estimates and the changing age structure of the population (Web Figure 3). We found that despite the aging population, the seroprevalence level has progressively decreased, from above 60% in the 1970s to 46% in 2013 (Figure 5B), though the gradient of the decrease has become more gradual since the mid-2000s. These results suggest that the change in age structure cannot fully explain the reduction in FOI.
To understand the observed discrepancy between the rising incidence rate and the reduced transmission rates, we sought to understand the trend of the infection:disease notification ratio in Singapore. Among the 4 serosurveys, the ratio declined from 14:1 (95% credible interval (CrI): 12:1, 16:1) between 2005 and 2009 to 8:1 between 2010 and 2013 (95% CrI: 6:1, 10:1) and subsequently 6:1 between 2014 and 2017 (95% CrI: 4:1, 8:1). Interestingly, during 2007–2012 and 2015–2017, when DENV-2 was the predominant serotype, the ratios were 16:1 (95% CrI: 14:1, 19:1) and 7:1 (95% CrI: 5:1, 10:1), respectively, whereas in 2005–2006 and 2013–2014, when DENV-1 predominated, the ratios were 10:1 (95% CrI: 8:1, 11:1) and 3:1 (95% CrI: 2:1, 5:1), respectively.
Estimated number of primary infections averted
Trends of primary dengue infections averted among residents in 2017 across age were projected from 4 hypothetical FOI situations (Figure 6). Assuming the number of primary and subsequent infections to be equal throughout the period, using estimates from patients presenting at primary-care clinics (27), estimates of the total number of infections averted would be 3.12, 1.90, 0.87, and −0.11 million for an FOI held fixed at the average of the 1960s, 1970s, 1980s, and 1990s, respectively. However, these 4 estimates are sensitive to the assumed ratio of primary infections to nonprimary infections and, in sensitivity analysis, vary over the range of 2.34, 1.43, 0.65, and −0.08 million to 4.68, 2.86, 1.30, and −0.17 million, if the ratio of primary cases to nonprimary cases is changed to 2:1 or 1:2. No additional infections were averted among residents on the basis of the FOI held fixed at the 2000s average, and a wide Bayesian credible interval was observed (–10,000 infections, 95% BCrI: −110,000, 100,000).
Figure 6.
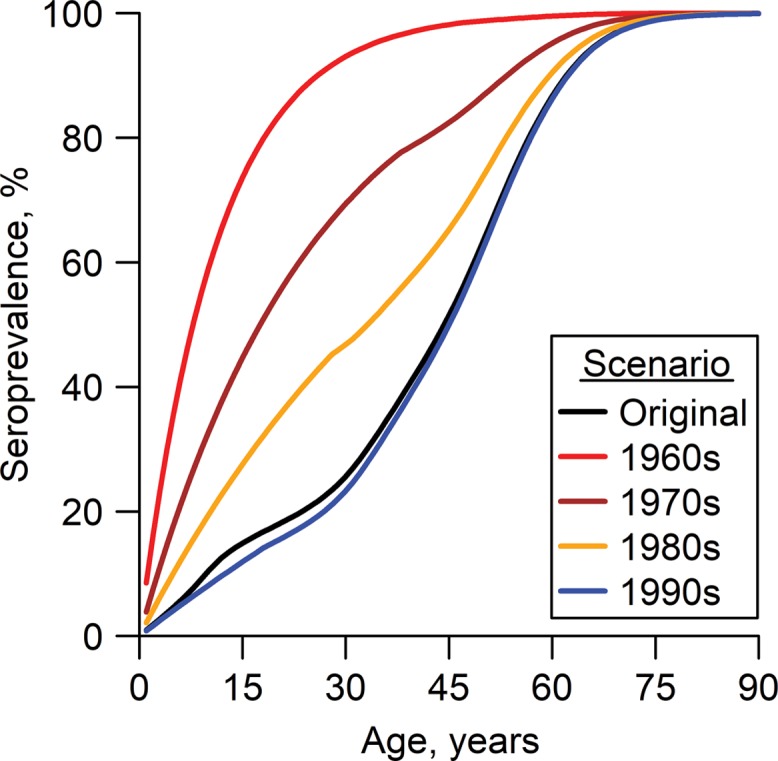
Estimated age-specific dengue seroprevalence in Singapore in 2017, modeled from 4 hypothetical circumstances: force of infection (FOI) fixed at the 1960s, 1970s, 1980s, and 1990s averages, respectively. The black line represents the age-specific seroprevalence in 2017 based on the original FOI estimates. Of the 4 hypothetical situations, the largest number of dengue infections averted occurs when the FOI is held fixed at the 1960s average (3.12 million; 95% Bayesian credible interval (BCrI): 2.94, 3.27), assuming that numbers of primary and subsequent dengue infections are similar. For scenarios where FOI is held fixed at the 1970s, 1980s, and 1990s averages, the numbers of dengue infections averted are estimated to be 1.90 million (95% BCrI: 1.63, 2.14), 0.87 million (95% BCrI: 0.61, 1.13), and −0.11 million (95% BCrI: −0.30, 0.07), respectively.
DISCUSSION
Regular seroprevalence studies in Singapore, coupled with mathematical models, have allowed estimation of FOI and the true number of dengue infections in this dengue-endemic city-state. The FOI reflects changes in the demographic characteristics of a population as well as the risk in transmission, and it can behave in nonintuitive ways; for instance, a falling FOI can coincide with a rise in the incidence of severe dengue (31). Cross-sectional serological studies coupled with epidemiologic models provide new insights beyond what is available from case notification alone: Marked changes in the FOI for dengue have been noted in Thailand, for instance, using pediatric cross-sections, mirroring a shift in the age profile of cases there (32).
This study shows that Singapore experienced a significant reduction in the dengue FOI between 1960 and 1990, followed by a steadily low FOI. The phenomenon is corroborated by the estimates for the same period. The estimate in Singapore is lower than estimates reported in other endemic places. For example, has been estimated to be 1.35 or more in Thailand, Sri Lanka, and Brazil (26). A reduction in FOI could be due to an aging population in which the birth rate decreased very significantly and life expectancy increased, resulting in increased herd immunity due to fewer susceptible new births and a concurrent increase in immune individuals as a proportion of the population (33). Indeed, Singapore has seen a rapidly aging population in the last 4 decades. However, there has been a reduction in seroprevalence in Singapore despite the changing age structure. Not surprisingly, the relatively small reduction in the overall prevalence of dengue does not corroborate with the large drop in age-specific prevalence seen through the same period. Together, these findings suggest that the lowering of the transmission rate indicated by FOI estimates is not mainly driven by a change in age structure, that the reduction in the FOI cannot solely be attributed to changing demographics, and that the evolving age structure may have prevented a more drastic decrease in seroprevalence. Singapore’s stringent vector prevention and control regime, which has continued to evolve to respond to the increasing challenge and involves the community in reducing levels of the vector (34), is one possible explanation for some of the reduction in transmission risk.
While the dengue incidence rate in Singapore has increased dramatically in the last 25 years, the trend contradicts the falling trend of FOI and seroprevalence. We suspect that this is largely due to the surfacing of a larger part of the dengue “iceberg” in the last 2 decades and to the availability of improved diagnostic procedures, increased awareness among the public, and increased use of laboratory diagnosis by the medical community. Besides the ongoing effort in promoting early laboratory testing and use of rapid diagnostic test kits in Singapore, the improved reporting rate could also be attributed to the shift of infections to adult age groups (discussed below), where primary dengue tends to be more apparent than it is in children (35). The improved ratio of total infections to notified cases provides evidence for the hypothesis that a higher rate of detection of infections is contributing to the increasing incidence rate.
Although we attribute much of the decline in to vector control, the decreases in transmission intensity may reflect both demographic changes that reduce transmission potential (a top-heavy age pyramid) and the intensive vector control program in Singapore, which has reduced mosquito breeding rates to very low levels. Our study has highlighted the importance of using seroprevalence data to evaluate the impact and efficacy of a vector control program and to estimate the number of potential cases averted. The measure “number of infections averted” has been widely applied in the evaluation of malaria disease control efforts in Africa (36) and the dengue vaccination program in Thailand (37), as well as potential dengue control tools such as the sterile insect technique (38). This point of reference is essential in the Singapore setting because the Aedes house index is low (approximately 0.3% in 2013) (18), and thus measurement of incidence does not reflect the effectiveness of control efforts well.
Since the 1980s, the lowering of herd immunity in the population has coincided with an increase in the median age of dengue infection, from 14 years in 1973 (39) to over 30 years in 2007 (40). While the low level of herd immunity has rendered the population vulnerable to epidemics despite a low Aedes aegypti population (18), the associated reduced dengue transmission has produced many benefits, with few pediatric cases and thus fewer children suffering from antibody-dependent enhancement inducing severe secondary infection (41).
However, the progressive lowering of herd immunity poses challenges. First, lower herd immunity makes vector control less effective, since each infected mosquito has a relatively greater chance of infecting 1 or more susceptible hosts. The need to further reduce the already-low vector population is expected to escalate costs considerably. A paradigm shift is needed to reduce contact between susceptible human hosts and infected vectors. Besides dengue vaccination (42), the use of Wolbachia-carrying Aedes (43) to further suppress the Aedes population could be considered. Second, the shift in the age of dengue cases has increasingly led to many infections in the elderly. In 2006, 2007, and 2009, persons aged 55 years or more experienced the highest incidence rate among all age groups (17, 44, 45). This, combined with the increased risk of severity among populations with comorbidity (41) and an aging population in Singapore (46), may pose a challenge to clinical management in the near future.
One limitation of this study was the use of residual sera from blood donors. To render the samples as representative of the Singapore resident population as possible, we adjusted for age. Analysis of the spatial distribution of the study subjects showed that they were representative of the resident population as a whole (Web Figure 4) and resided in the two most common types of housing in Singapore (public and private high-rise apartment buildings). We believe that the spatial distribution of risk in Singapore is probably more uniform than that in the majority of other dengue-endemic countries. This is supported by a previous serological study which indicated that housing type was not significantly associated with previous infection (24) and by the fact that incidence rates between these two common housing types are similar (47). Other evidence supporting our use of blood donors’ samples includes the similar seroprevalence results and FOI estimates obtained from this study as compared with those derived from the 2004 survey (Web Figure 1). Unfortunately, information on ethnicity was not collected, and thus we could not ascertain whether blood donors matched the population as a whole in this regard. Bias may have been introduced by the request that donors with recent (diagnosed) dengue or chikungunya virus infection defer their donation for 6 months (48), which may have affected the 2013 estimates in particular, because of the large dengue outbreak that year. Lastly, the temporal FOI model was based on non–serotype-specific data, given the limitation of the IgG ELISA used in this study and most of the prevalence studies.
Despite these limitations, this work highlights how dengue has evolved over time in Singapore and that a substantial proportion of children and young adults are susceptible to dengue infection. The sensitivity of the national notification system, which relies on diagnosed cases, may vary over time, and hence the magnitudes of epidemics are not comparable across calendar years. Our findings provide updated estimates of overall dengue transmission intensity and modeled primary infections averted that could help guide analyses of the potential impact of future disease interventions or vaccination programs.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Environmental Health Institute, National Environment Agency, Singapore (Li Kiang Tan, Swee Ling Low, Yuan Shi, Lilac Liu, Wing Yan Wong, Rachel Chua, Lee Ching Ng); Saw Swee Hock School of Public Health, National University of Singapore and National University Health System, Singapore (Haoyang Sun, Alex R. Cook); Blood Services Group, Health Sciences Authority, Singapore (Sally Lam, Hwee Huang Tan, Diana Teo); Epidemiology and Disease Control Division, Ministry of Health, Singapore (Li Wei Ang); and School of Biological Sciences, Nanyang Technological University, Singapore (Lee Ching Ng).
L.K.T and S.L.L. contributed equally to this paper.
This study was supported by the National Environment Agency of Singapore. A.R.C. was supported by the Singapore Ministry of Health’s National Medical Research Council under the Centre Grant Programme of the Singapore Population Health Improvement Centre (grant NMRC/CG/C026/2017_NUHS).
We thank Prof. Neil Ferguson of the Faculty of Medicine, Imperial College London (London, United Kingdom), for his technical advice on statistical modeling and Janet Ong and Jayanthi Rajarethinam of the National Environment Agency (Singapore) for information and support. We also thank Sze Sze Chua and Kooi Sim Ng of the Blood Services Group, Health Sciences Authority (Singapore), for their assistance with consolidation of samples.
Conflict of interest: none declared.
Abbreviations
- BCrI
Bayesian credible interval
- CI
confidence interval
- CrI
credible interval
- DENV-1
dengue virus type 1
- DENV-2
dengue virus type 2
- ELISA
enzyme-linked immunosorbent assay
- FOI
force of infection
- IgG
immunoglobulin G.
REFERENCES
- 1. World Health Organization Dengue and severe dengue. (Fact sheet no. 117). 2017. http://www.who.int/mediacentre/factsheets/fs117/en/. Accessed October 30, 2017.
- 2. World Health Organization Global Strategy for Dengue Prevention and Control, 2012–2020 Geneva, Switzerland: World Health Organization; 2012. http://www.who.int/denguecontrol/9789241504034/en. Accessed October 30, 2017.
- 3. Suaya JA, Shepard DS, Beatty ME Dengue: burden of disease and costs of illness. (Working paper 32). In: Report of the Scientific Working Group on Dengue, Geneva, 1–5 October 2006 Geneva, Switzerland: World Health Organization; 2007:35–49.
- 4. Standish K, Kuan G, Avilés W, et al. . High dengue case capture rate in four years of a cohort study in Nicaragua compared with national surveillance data. PLoS Negl Trop Dis. 2010;4(3):e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wichmann O, Yoon IK, Vong S, et al. . Dengue in Thailand and Cambodia: an assessment of the degree of underrecognized disease burden based on reported cases. PLoS Negl Trop Dis. 2011;5(3):e996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Endy TP, Chunsuttiwat S, Nisalak A, et al. . Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156(1):40–51. [DOI] [PubMed] [Google Scholar]
- 7. Balmaseda A, Hammond SN, Tellez Y, et al. . High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop Med Int Health. 2006;11(6):935–942. [DOI] [PubMed] [Google Scholar]
- 8. Gordon A, Kuan G, Mercado JC, et al. . The Nicaraguan Pediatric Dengue Cohort Study: incidence of inapparent and symptomatic dengue virus infections, 2004–2010. PLoS Negl Trop Dis. 2013;7(9):e2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burke DS, Nisalak A, Johnson DE, et al. . A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38(1):172–180. [DOI] [PubMed] [Google Scholar]
- 10. Bhatt S, Gething PW, Brady OJ, et al. . The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control Geneva, Switzerland: World Health Organization; 2009. http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf. Accessed October 30, 2017.
- 12. Goh KT, Yamazaki S. Serological survey on dengue virus infection in Singapore. Trans R Soc Trop Med Hyg. 1987;81(4):687–689. [DOI] [PubMed] [Google Scholar]
- 13. Committee on Epidemic Diseases, Singapore Ministry of Health Surveillance of dengue fever/dengue haemorrhagic fever, 1992. Epidemiol News Bull. 1993;19:13–17. [Google Scholar]
- 14. Committee on Epidemic Diseases, Singapore Minstry of Health Dengue surveillance in Singapore, 1998. Epidemiol News Bull. 1999;25:1–3. [Google Scholar]
- 15. Singapore Ministry of Health Dengue fever/dengue haemorrhagic fever (DF/DHF). In: Communicable Diseases Surveillance in Singapore 2005 Singapore: Ministry of Health; 2006:25–37. https://www.moh.gov.sg/docs/librariesprovider5/resources-statistics/reports/vector_borne_diseases.pdf. Accessed November 1, 2018.
- 16. Singapore Ministry of Health Dengue fever/dengue haemorrhagic fever. In: Communicable Diseases Surveillance in Singapore 2004 Singapore: Ministry of Health; 2005:27–38. https://www.moh.gov.sg/docs/librariesprovider5/resources-statistics/reports/vector-borne_diseases.pdf. Accessed November 1, 2018.
- 17. Singapore Ministry of Health Dengue fever/dengue haemorrhagic fever (DF/DHF). In: Communicable Diseases Surveillance in Singapore 2007 Singapore: Ministry of Health; 2008:22–33. https://www.moh.gov.sg/docs/librariesprovider5/resources-statistics/reports/vector-borne-diseasesfd4c6200e7f04f3680bdd172c387953a.pdf. Accessed November 1, 2018.
- 18. Singapore Ministry of Health Dengue fever/dengue haemorrhagic fever (DF/DHF). In: Communicable Diseases Surveillance in Singapore 2013 Singapore: Ministry of Health; 2014:52–65. https://www.moh.gov.sg/docs/librariesprovider5/resources-statistics/reports/vector-borne--zoonotic-diseases.pdf. Accessed November 1, 2018.
- 19. Hapuarachchi HC, Koo C, Kek R, et al. . Intra-epidemic evolutionary dynamics of a dengue virus type 1 population reveal mutant spectra that correlate with disease transmission. Sci Rep. 2016;6:22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hapuarachchi HC, Koo C, Rajarethinam J, et al. . Epidemic resurgence of dengue fever in Singapore in 2013–2014: a virological and entomological perspective. BMC Infect Dis. 2016;16:Article 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Low SL, Lam S, Wong WY, et al. . Dengue seroprevalence of healthy adults in Singapore: serosurvey among blood donors, 2009. Am J Trop Med Hyg. 2015;93(1):40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reiner RC Jr, Stoddard ST, Forshey BM, et al. . Time-varying, serotype-specific force of infection of dengue virus. Proc Natl Acad Sci U S A. 2014;111(26):E2694–E2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egger JR, Ooi EE, Kelly DW, et al. . Reconstructing historical changes in the force of infection of dengue fever in Singapore: implications for surveillance and control. Bull World Health Organ. 2008;86(3):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yew YW, Ye T, Ang LW, et al. . Seroepidemiology of dengue virus infection among adults in Singapore. Ann Acad Med Singapore. 2009;38(8):667–675. [PubMed] [Google Scholar]
- 25. Ang LW, Cutter J, James L, et al. . Seroprevalence of past dengue virus infection among children and adolescents in Singapore. J Med Virol. 2015;87(12):2159–2162. [DOI] [PubMed] [Google Scholar]
- 26. Imai N, Dorigatti I, Cauchemez S, et al. . Estimating dengue transmission intensity from sero-prevalence surveys in multiple countries. PLoS Negl Trop Dis. 2015;9(4):e0003719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Low JG, Ooi EE, Tolfvenstam T, et al. . Early Dengue Infection and Outcome Study (EDEN)—study design and preliminary findings. Ann Acad Med Singapore. 2006;35(11):783–789. [PubMed] [Google Scholar]
- 28. Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. [DOI] [PubMed] [Google Scholar]
- 29. R CoreTeam R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 30. Ang LW, Cutter J, James L, et al. . Seroepidemiology of dengue virus infection in the adult population in tropical Singapore. Epidemiol Infect. 2015;143(8):1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagao Y, Koelle K. Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever. Proc Natl Acad Sci U S A. 2008;105(6):2238–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodríguez-Barraquer I, Buathong R, Iamsirithaworn S, et al. . Revisiting Rayong: shifting seroprofiles of dengue in Thailand and their implications for transmission and control. Am J Epidemiol. 2014;179(3):353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cummings DA, Iamsirithaworn S, Lessler JT, et al. . The impact of the demographic transition on dengue in Thailand: insights from a statistical analysis and mathematical modeling. PLoS Med. 2009;6(9):e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ng LC, Tan HK, Tan LK, et al. . Evolving dengue control programme in Singapore. Epidemiol News Bull. 2016;42(1):11–15. [Google Scholar]
- 35. Trung DT, Thao le TT, Dung NM, et al. . Clinical features of dengue in a large Vietnamese cohort: intrinsically lower platelet counts and greater risk for bleeding in adults than children. PLoS Negl Trop Dis. 2012;6(6):e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhatt S, Weiss DJ, Cameron E, et al. . The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chao DL, Halstead SB, Halloran ME, et al. . Controlling dengue with vaccines in Thailand. PLoS Negl Trop Dis. 2012;6(10):e1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alphey N, Alphey L, Bonsall MB. A model framework to estimate impact and cost of genetics-based sterile insect methods for dengue vector control. PLoS One. 2011;6(10):e25384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goh KT. Changing epidemiology of dengue in Singapore [letter]. Lancet. 1995;346(8982):1098. [DOI] [PubMed] [Google Scholar]
- 40. Ler TS, Ang LW, Yap GS, et al. . Epidemiological characteristics of the 2005 and 2007 dengue epidemics in Singapore—similarities and distinctions. Western Pac Surveill Response J. 2011;2(2):24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rowe EK, Leo YS, Wong JG, et al. . Challenges in dengue fever in the elderly: atypical presentation and risk of severe dengue and hospital-acquired infection. PLoS Negl Trop Dis. 2014;8(4):e2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schwartz LM, Halloran ME, Durbin AP, et al. . The dengue vaccine pipeline: implications for the future of dengue control. Vaccine. 2015;33(29):3293–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoffmann AA, Montgomery BL, Popovici J, et al. . Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454–457. [DOI] [PubMed] [Google Scholar]
- 44. Singapore Ministry of Health Dengue fever/dengue haemorrhagic fever (DF/DHF). In: Communicable Diseases Surveillance in Singapore 2006 Singapore: Ministry of Health; 2007:25–35. https://www.moh.gov.sg/docs/librariesprovider5/resources-statistics/reports/vector-borne-diseases(3).pdf. Accessed November 1, 2018.
- 45. Singapore Ministry of Health Dengue fever/dengue haemorrhagic fever (DF/DHF). In: Communicable Diseases Surveillance in Singapore 2009 Singapore: Ministry of Health; 2010:24–39. https://www.moh.gov.sg/docs/librariesprovider5/resources-statistics/reports/vector-borne-2009.pdf. Accessed November 1, 2018.
- 46. Low JG, Ooi EE. Dengue—old disease, new challenges in an ageing population. Ann Acad Med Singapore. 2013;42(8):373–375. [PubMed] [Google Scholar]
- 47. Singapore Ministry of Health Dengue fever/dengue haemorrhagic fever (DF/DHF). In: Communicable Diseases Surveillance in Singapore 2014 Singapore: Ministry of Health; 2015:53–66. https://www.moh.gov.sg/docs/librariesprovider5/resources-statistics/reports/vector-borne-zoonotic-diseasesb061cae8c6ef49cd98e8869bb00033c6.pdf. Accessed November 1, 2018.
- 48. Teo D, Ng LC, Lam S. Is dengue a threat to the blood supply? Transfus Med. 2009;19(2):66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.