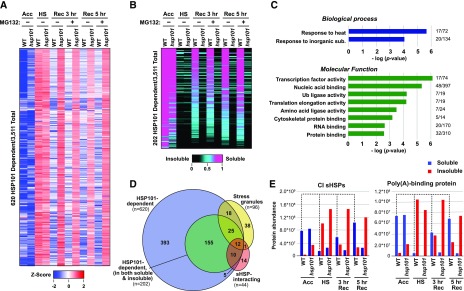
Figure 6.
HSP101 disaggregates numerous proteins after heat stress, which are predominantly reconstituted in the soluble fraction during recovery. Two-week-old wild-type (WT) and hsp101 seedlings were subjected to the same treatments as reported in Figure 5, including a 3- and 5-h recovery time point. Total extracts prepared from seedlings harvested after the various heat stress time points were separated into soluble and insoluble/aggregated fractions and subjected to tandem MS using the MS1 precursor ion intensities for label-free quantification. A, A large collection of proteins that aggregate during heat stress are retained in the insoluble fraction in the absence of HSP101. Shown is the abundance of 620 proteins in wild-type and hsp101 seedlings (from a total of 3,511 detected proteins [FDR < 5%]), that were at least 4-fold more abundant in the insoluble fraction after heat stress (HS) as compared to the acclimated (Acc) samples, and showed a subsequent 50% decrease in the insoluble fraction in the wild type, but not in hsp101 samples, during recovery. Shown are their dynamics displayed by a heat map based on Z-scores and ranked by unrooted hierarchical clustering. Blue and red indicate a lower and higher abundance for each protein in the insoluble fraction, respectively. B, HSP101 has a dramatic effect on the solubility of numerous proteins. From the dataset of insoluble proteins identified in A, 202 proteins were selected that were consistently detected in all insoluble and soluble samples from the wild type. Their solubility was calculated by their ratio in the soluble/insoluble fractions after the 45°C heat stress and then ranked by dependency on HSP101, using a heat map to visualize solubility. C, HSP101 client proteins identified in B are enriched in various biological processes and molecular functions. Gene ontology (GO) enrichment was conducted using a singular enrichment analysis. P-values of the most significant and unique GO-terms regarding biological processes and molecular functions were −log transformed and displayed in bar graphs. The numbers at the end of each bar reflect the number of proteins identified compared to the total number of proteins in each GO category. D, Venn diagram showing the overlap between HSP101 clients as determined from the insoluble fraction (as in A), HSP101 clients detected in both the soluble and insoluble fractions (as in B), stress granule constituents, and sHSP-interacting proteins. The number of proteins in each sector is indicated. E, Dynamics of representative HSP101 clients during heat stress and recovery. Shown are the abundance CI sHSPs and poly(A) binding proteins in the soluble and insoluble protein fractions from wild-type and hsp101 seedlings after the indicated stages of the heat stress regime. CI sHSPs and the stress granule marker poly(A) binding protein showed strong partitioning into the insoluble fraction upon heat stress and then returned to the soluble fraction during recovery by a process dependent on HSP101. The dashed lines highlight protein abundance in the soluble fraction of the wild-type samples. The data in this figure are from one representative of three biological replicates.