Figure 15.
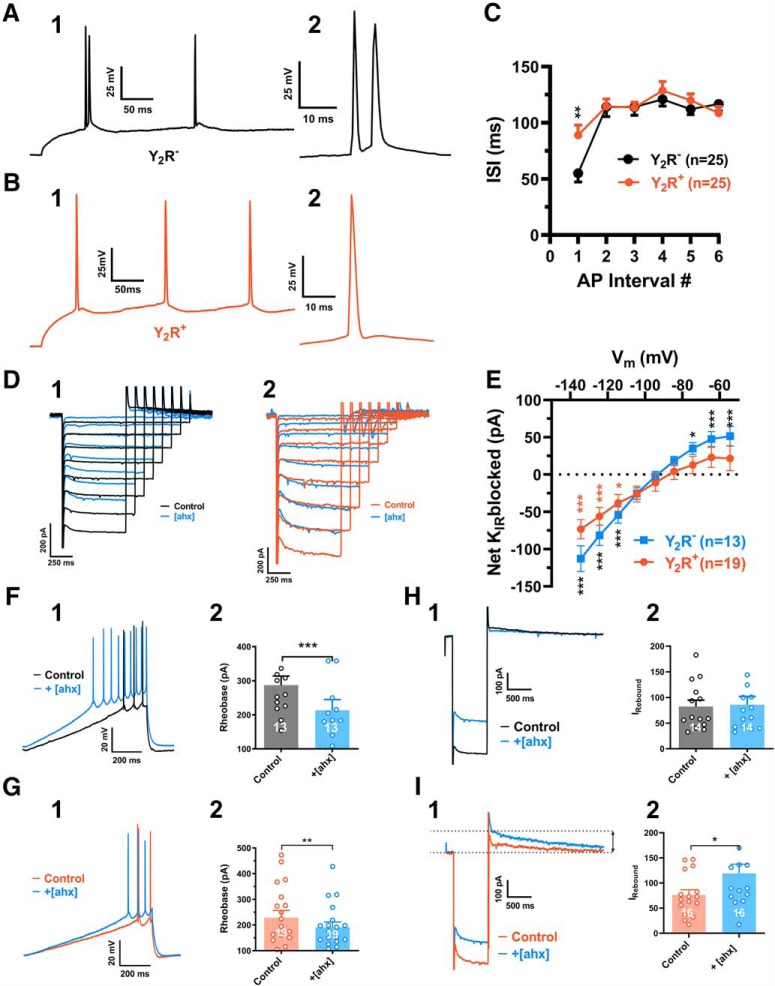
Y2R-mediated responses in Y2R+ and Y2R− BLA PNs in Y2R-tdTomato mice. A1, An AP train evoked in a nonfluorescent PN in BLA of a Y2R-tdTomato mouse, with an initial AP doublet burst. A2, Shown on a faster timescale, initial doublet APs (interspike interval < 10 ms) were relatively common (7 of 25 tested) in nonfluorescent PNs. B1, An AP train evoked in a fluorescent (Y2R-expressing; orange) PN in the BLA of a Y2R-tdTomato mouse. B2, Shown at a faster timescale, the first AP of this train. Initial AP doublets (as in A) were less common (3 of 25 tested) in fluorescent PNs. C, AP interspike intervals, plotted against position in a train, from fluorescent (n = 25 cells, 18 mice; orange) and nonfluorescent (n = 25 cells, 12 mice; black) BLA PNs from Y2R-tdTomato mice (two-way ANOVA; cell type: F(1,304) = 0.6109, p = 0.4350; AP number: F(8,304) = 10.44, p < 0.0001; interaction: F(8,304) = 1.718, p = 0.0936). The first interspike interval was significantly shorter in nonfluorescent than in fluorescent PNs (54.9 ± 7.7 ms vs 88.9 ± 9.0 ms; p = 0.0023, Bonferroni's post-test). D1, Current responses to hyperpolarizing voltage steps from a representative nonfluorescent Y2R-tdTomato mouse BLA PN, in control (black) and in [ahx5–24]NPY (1 μm) (blue). D2, Current responses from a fluorescent BLA PN in a Y2R-tdTomato mouse, in control (orange) and in [ahx5–24]NPY (1 μm) (blue). E, KIR current blocked by [ahx5–24]NPY (1 μm) in nonfluorescent PNs (blue; n = 13) and fluorescent PNs (orange; n = 19) in BLA from Y2R-tdTomato mice. The Y2R agonist significantly reduced the KIR current in both fluorescent and nonfluorescent PNs. Nonfluorescent PNs: two-way repeated-measures ANOVA; treatment: F(1,108) = 14.22, p = 0.0003; voltage: F(8,108) = 88.76, p < 0.0001; interaction: F(8,108) = 28.75, p < 0.0001; n = 13 cells, 10 mice. The Y2R agonist significantly reduced the KIR as indicated (Bonferroni's post-test). Fluorescent PNs: two-way repeated-measures ANOVA; treatment: F(1,162) = 14.45, p = 0.0002; voltage: F(8,165) = 111.7, p < 0.0001; interaction: F(8,165) = 7.762, p < 0.0001, n = 19 cells, 14 mice. The Y2R agonist significantly reduced the KIR as indicated (Bonferroni's post-test). F1, Responses of a nonfluorescent PN to a depolarizing current ramp from RMP in control (black) and in [ahx5–24]NPY (1 μm) (blue). F2, The Y2R agonist significantly reduced rheobase current in nonfluorescent PNs, from 287.1 ± 26.7 pA to 213 ± 31.8 pA (t(12) = 4.902; p < 0.0004; n = 13 cells, 10 mice; Student's paired t test). G1, Responses of a fluorescent PN to a depolarizing current ramp from RMP in control (orange) and in [ahx5–24]NPY (1 μm) (blue). G2, The Y2R agonist significantly reduced rheobase current in fluorescent PNs from 228.7 ± 27.7 pA to 190.9 ± 20.5 pA (t(18) = 2.878; p < 0.0100; n = 19 cells, 14 mice; Student's paired t test). H1, Response of a nonfluorescent PN to a hyperpolarizing voltage step (as in Fig. 9) in control (black) and in [ahx5–24]NPY (1 μm) (blue). Although the Y2R agonist substantially decreased the KIR in this PN, it did not enhance the IsAHP-mediated rebound outward current. H2, In nonfluorescent PNs, [ahx5–24]NPY (1 μm) did not enhance the rebound current (control: 82.8 ± 12.2 pA, [ahx]: 85.9 ± 16.2 (t(13) = 0.3176; p = 0.7558; n = 14 cells, 11 mice; Student's paired t test). I1, Response of a fluorescent PN to a hyperpolarizing voltage step in control (orange) and in [ahx5–24]NPY (1 μm) (blue). The Y2R agonist substantially decreased the KIR and enhanced the IsAHP-mediated rebound current. I2, In fluorescent PNs, [ahx5–24]NPY (1 μm) significantly increased the rebound current from 76.2 ± 10.5 pA to 119.1 ± 18.4 pA (t(15) = 2.878; p = 0.0115; n = 16 cells, 13 mice; Student's paired t test). *p < 0.05, **p < 0.01, ***p < 0.001.