Figure 1.
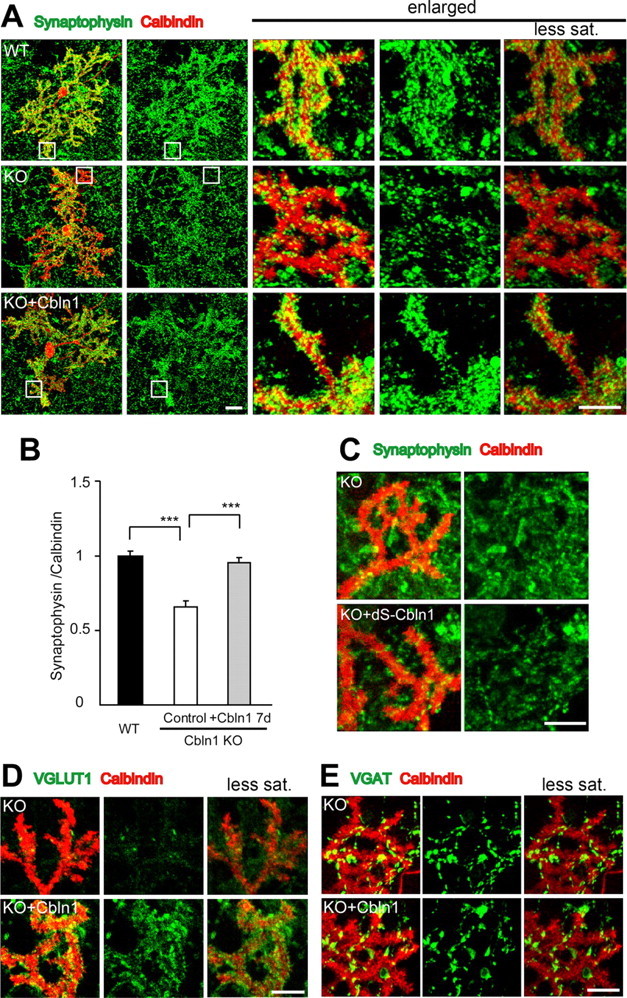
Excitatory synapse formation induced by exogenous Cbln1 in cultured Purkinje cells. A, Representative images of cultured Purkinje cells at 21 DIV, prepared from wild-type (WT) and cbln1-null mice [knock-out (KO)] and immunostained against calbindin (red) and synaptophysin (green). The three columns on the right show enlarged views of the boxed regions in the two left columns. The far right column shows the images taken with reduced gain and saturation (less sat.) settings. B, The intensity of synaptophysin immunoreactivity is strong at the distal dendrites in WT cells but significantly weaker in cbln1-null cells. Incubation with exogenous Cbln1 (3 μg/ml) in the medium for 7 d completely restored the reduction in the synaptophysin signal. The data were normalized to the averages of the wild-type control cells for each experiment (n = 24 cells; 3 independent experiments; ***p < 0.0001). C, Images of distal dendrites of cultured Purkinje cells stained with calbindin (red) and synaptophysin (green). Incubation with a mutant Cbln1 (dS-Cbln1; 3 μg/ml) did not change synaptophysin signals in cbln1-null Purkinje cells. D, E, Images of distal dendrites of cultured Purkinje cells stained with calbindin (red) and either VGluT1 (D, green) or VGAT (E, green). The far right column shows images taken with reduced gain and saturation settings. Application of Cbln1 to the medium of cbln1-null cells increased the signals of VGluT1 (D) but not of VGAT (E). Scale bars: A, two far left columns, 30 μm (the entire images of the Purkinje cell); A, three columns on the right, C–E, 10 μm (the enlarged view of the distal dendrites).
