Figure 4.
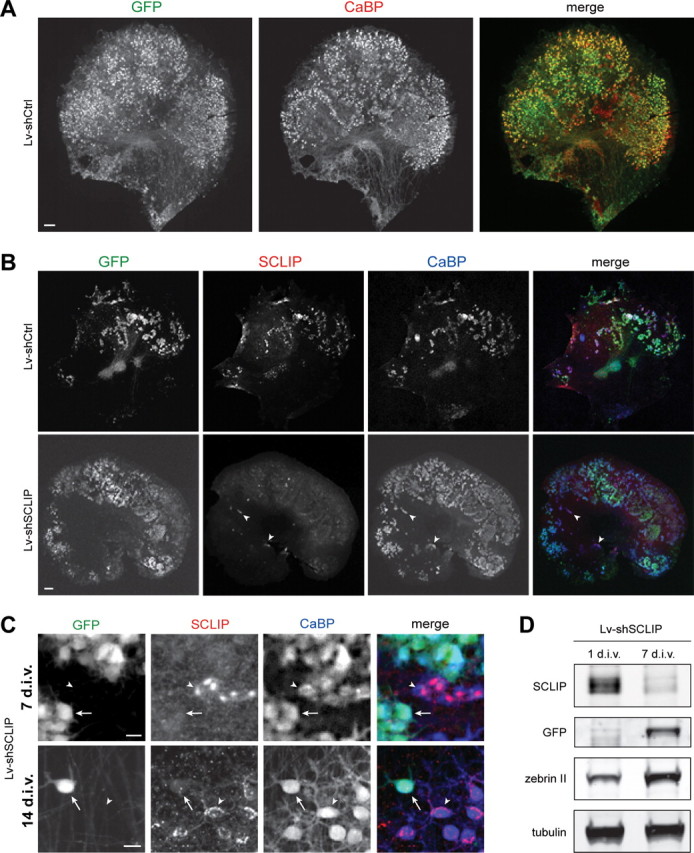
Lentiviral-mediated RNAi is adapted to study SCLIP function in PC dendritic development. A, Lentiviral-mediated transduction in organotypic cultures allows gene transfer in PCs. Neonatal cerebellar organotypic cultures were infected with Lv-shCtrl just after plating and immunostained for GFP (green) and CaBP (red) 14 d later. Among transduced cells visualized by GFP, numerous cells were identified as PCs, as revealed by colabeling with CaBP. Scale bar, 200 μm. B, Lentiviral-mediated RNAi inhibits SCLIP expression in PCs. Cerebellar organotypic cultures were infected with the Lv-shCtrl or the Lv-shSCLIP vectors just after plating and immunostained for GFP (green), SCLIP (red), and CaBP (blue) 7 d later. SCLIP labeling is no more observed in PCs transduced with Lv-shSCLIP (visualized by GFP), whereas it remains clearly detected in untransduced PCs (arrowheads) or Lv-shCtrl transduced PCs (top panels). Scale bar, 200 μm. C, Higher magnifications showing the inhibition of SCLIP expression in PCs transduced by Lv-shSCLIP after 7 and 14 div. At both 7 and 14 div, SCLIP labeling is no more detected in transduced PCs visualized by GFP (arrows), whereas it remains high in untransduced PCs (arrowheads). Scale bar, 20 μm. D, Western blot characterization of Lv-shSCLIP efficiency for inhibiting SCLIP expression. Cerebellar organotypic cultures were infected with the Lv-shSCLIP vector just after plating and protein expression was evaluated 1 and 7 d later. SCLIP expression is strongly inhibited at 7 div, whereas that of GFP concomitantly increases. In contrast, tubulin or zebrin (specific of PCs) expression is not significantly affected by the lentiviral-mediated RNAi treatment.
