Figure 3.
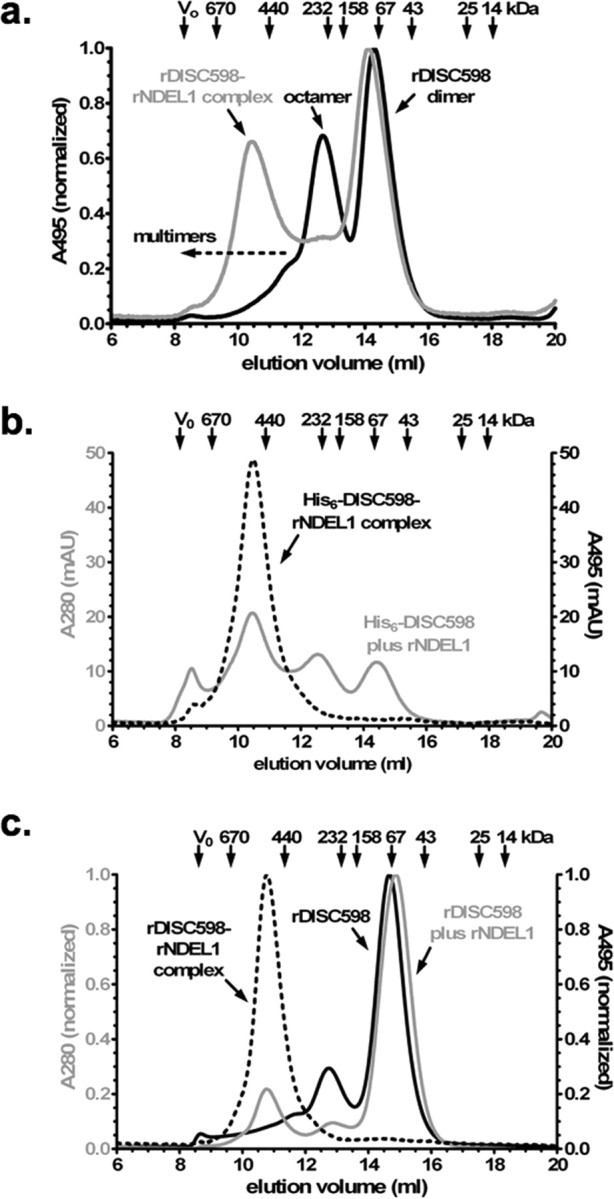
Analysis of interaction between rDISC598 and rNDEL1 by SEC. a, Normalized SEC profile at 495 nm of fluorescein-labeled rDISC598 alone (black) and combined with nonlabeled rNDEL1 (gray; 1:4 as monomer). The resulting complex peak (∼500 kDa) reflects a homogenous species, whereby the disappearance of the rDISC598 octamer peak indicates that NDEL1 preferentially binds the octamer and not the dimer (∼65 kDa) and is unlikely to bind multimers (>300 kDa). b, SEC profile at 280 nm (total protein; gray) and 495 nm of His6-tagged rDISC598 plus fluorescein-tagged NDEL1 (dashed black; 1:4), confirming that NDEL1 has little or no affinity for multimeric rDISC598. The presence of the hexahistidine tag increases favors multimerization without affecting overall structure, as verified by CD spectroscopy. c, Normalized SEC profile at 280 and 495 nm of nonlabeled DISC598 without (solid black) or with (gray; 10:1) fluorescein-labeled rNDEL1. Again, the rDISC598-rNDEL1 complex (495 nm; dashed black) is homogenous, even with an excess of rDISC598. The rDISC598 used here is the isolated and then concentrated dimer fraction, verifying that previously non-NDEL1-binding dimers can form NDEL1-binding octamers.
