Figure 7.
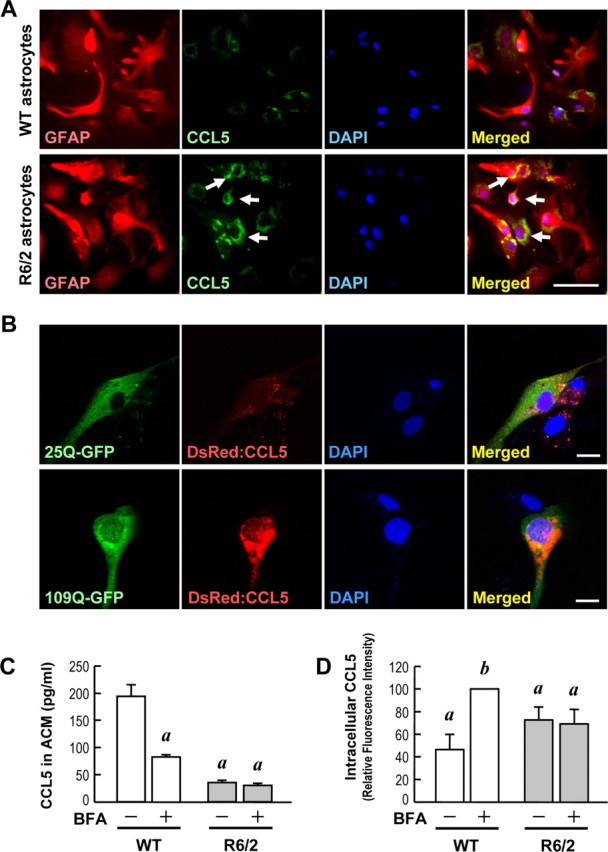
mHtt suppressed the release of CCL5/RANTES from primary astrocytes. A, Immunostaining of CCL5/RANTES (green) and GFAP (red) was performed in primary astrocytes. Nuclei were stained with Hoechst 33258 (blue). CCL5/RANTES was enriched inside of R6/2 astrocytes (bottom) but not in WT astrocytes (top). Scale bar, 100 μm. B, WT astrocytes were transfected with DsRed:CCL5 along with the Htt-(Q)25-hrGFP or Htt-(Q)109-hrGFP construct at a ratio of 1:1 for 48 h. Cells were fixed, and the fluorescent signals were detected using confocal microscopy. Scale bars, 20 μm. C, D, Both WT and R6/2 astrocytes were treated with either 0.1% DMSO as a control or BFA (10 μg/ml) for 4 h to block secretion of any newly synthesized CCL5/RANTES protein. Intracellular CCL5/RANTES levels were assessed by immunocytochemical staining using an anti-CCL5/RANTES. CCL5/RANTES accumulation was enhanced by BFA in WT astrocytes, but not in R6/2 astrocytes, as shown in supplemental Figure S4 (available at www.jneurosci.org as supplemental material). C, CCL5/RANTES released extracellularly from astrocytes was collected and detected using a CCL5/RANTES ELISA assay. D, The relative CCL5/RANTES fluorescence intensities of both vehicle- and BFA-treated WT and R6/2 astrocytes were quantified. Results are given as the mean ± SEM from three independent assays and normalized to the CCL5/RANTES intensity in BFA-treated WT astrocytes. Approximately 1000 cells were quantified in each group. ap < 0.001 compared with the DMSO-treated control in WT astrocytes; bp < 0.001 compared with the BFA-treated WT astrocytes by Student's t test.
