Figure 1.
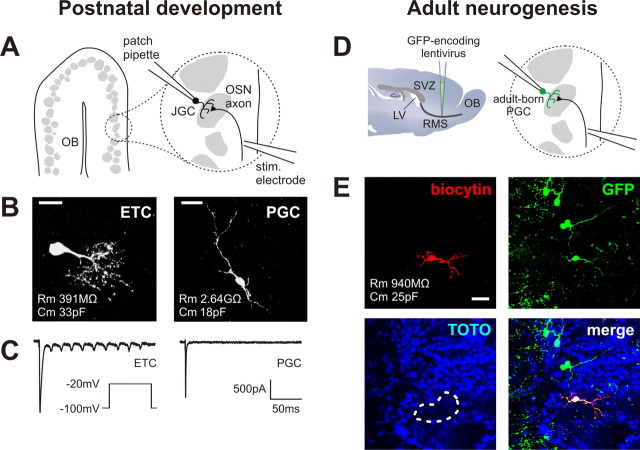
Recording evoked olfactory nerve inputs in three types of maturing OB glomerular layer neurons. A, Schematic of our experimental approach for developing postnatal juxtaglomerular cells (JGCs). Acute horizontal slices were cut from mouse olfactory bulb, and a monopolar stimulating electrode was placed in the olfactory nerve layer. Evoked inputs from OSN axons were then recorded via a patch pipette placed on a juxtaglomerular cell. B, Biocytin fills illustrating the two types of developing juxtaglomerular cell recorded. ETCs were large, with correspondingly low Rm and high Cm. PGCs were much smaller, with high Rm and low Cs. Scale bar, 20 μm. C, Sodium currents recorded in an ETC (left) and a PGC (right) after depolarization to −20 mV. Whereas ETCs displayed multiple currents, PGCs displayed only one. D, Schematic of our experimental approach for adult-born periglomerular cells. Newly born cells migrating toward the OB were labeled in adult mice via stereotaxic injection of a GFP-expressing lentivirus into the RMS. We then patched GFP+ cells in the glomerular layer and recorded evoked olfactory nerve inputs as shown above. LV, Lateral ventricle. E, An example of a recorded adult-born PGC. The biocytin fill (red) showed typical PGC morphology, whereas subsequent immunohistochemical staining for GFP (green) showed that the recorded cell was indeed newly generated. TOTO (blue) labels cell bodies and shows glomerular structure; the recorded cell's glomerulus is outlined in white. Scale bar, 20 μm.