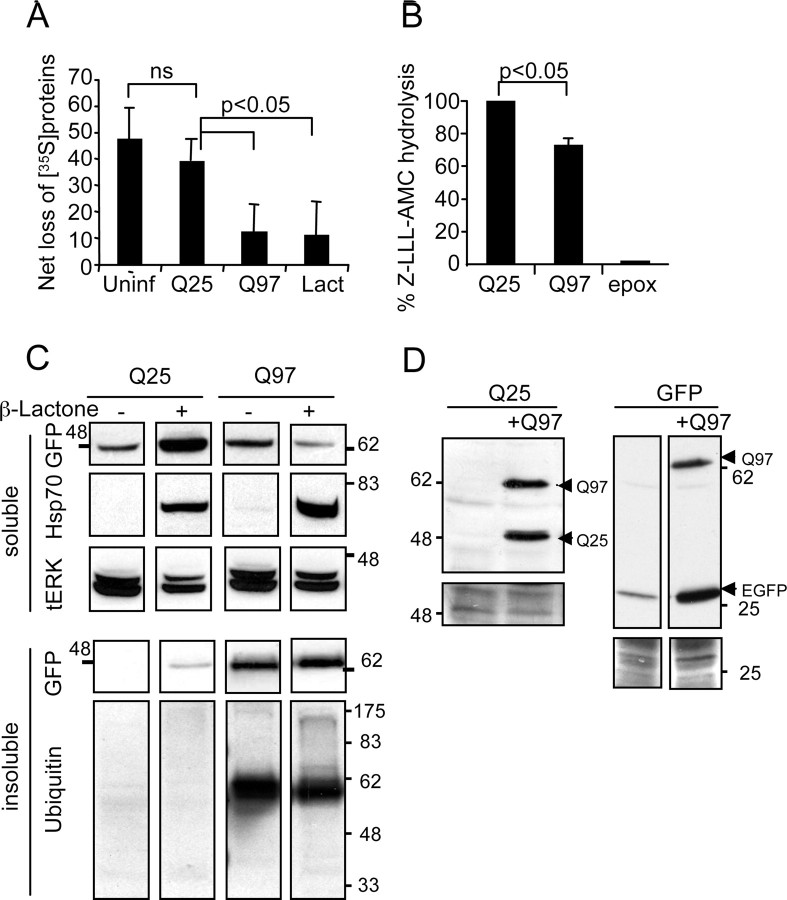
Figure 7.
Q97 inhibits proteasomal activity and function. A, Measurement of protein degradation. SCG cultures were infected with Q25 or Q97 for 2 d, whereas uninfected cohort cultures were either left untreated or treated with 10 μm lactacystin for 12 h before the four sets of cultures were washed free of methionine and labeled with [35S]methionine and described in Materials and Methods. After 1 h, cells were washed, medium was replenished with unlabeled methionine, and the amount of TCA-precipitable 35S-labeled protein remaining in the neurons was determined after 1 h or 11 h of incubation. Results show the proportion of label retained in the neurons 11 h after onset of chase normalized to values obtained after 1 h [mean ± SD; n = 4, Q25, Q97; n = 3 uninfected (Uninf) or lactacystin (Lact) treated; p < 0.012 by ANOVA; pairwise comparisons by t test]. B, Proteasomal activity assay. Neurons expressing Q25 or Q97 for 2 d were extracted in proteasome assay buffer, normalized for the amount of protein, and incubated for 30 h with z-LLL-AMC. Control neurons were treated with 20 μm epoxomycin (epox) over the same period to measure maximal activity. Activity was measured in a fluorescent plate reader (mean ± range; n = 2; p < 0.05, one-sample t test). C, Proteasome inhibition causes accumulation of Q25 and Hsp70. Neurons were infected with Ad-Q25 or Ad-Q97 and after 90 min were treated with 4 μm β-lactone when indicated for 20 h. Soluble fractions were analyzed by immunoblotting for expression of Q25/Q97 with anti-GFP or Hsp70 expression. Loading was controlled with anti-tERK1/2. Note accumulation of Q25 and Hsp70 in the soluble fraction in β-lactone-treated neurons but no parallel increase in the amount of Q97. The insoluble fraction was probed with anti-GFP and anti-ubiquitin. Note no change in the amount of Q97 or ubiquitylated Q97 in β-lactone-treated neurons. D, Q97 causes accumulation of Q25 or GFP. SCG neurons were infected with Ad-Q25 or Ad-GFP alone or coinfected with Ad-Q97 and cultured without additional treatment for 24 h. Soluble fractions were analyzed by immunoblotting with anti-GFP antibodies. Note increased amounts of Q25 or EGFP only under conditions in which Q97 was coexpressed. Bottom panels show actin and Ponceau S staining as loading controls.