Figure 1.
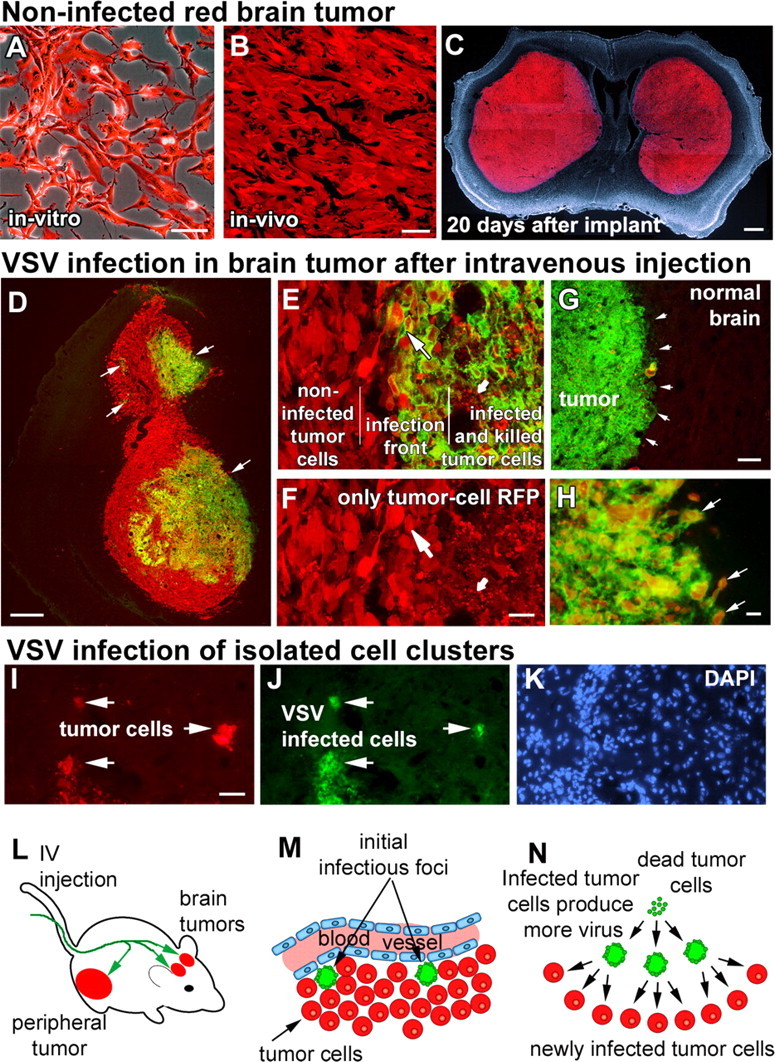
Intravenously injected VSVrp30a infects intracranial xenografts at multiple sites and spreads within the tumor to infect the tumor mass and infiltrating tumor cells. Stably RFP-transfected and fluorescence-activated cell-sorted U87MG human high-grade glioma cells grew in vitro without any signs of cytotoxicity (A) and formed brain tumors with no signs of spontaneous regression (B), eventually resulting in the animal's demise (C). When tumor-bearing animals were given a single intravenous injection of VSVrp30a, multiple independent foci (arrows in D) of VSV infection were found inside the tumor mass. Tumor cells in infected areas demonstrated marked cytopathy, and most were lysed (small arrow in E, F). Viral progeny released from these initially infected cells spread to neighboring tumor cells (large arrow in E, F) creating distinct areas of cells in different phases of VSV infection. In addition to infecting the whole tumor, VSVrp30a also infected individual infiltrative tumor cells close to the tumor mass (arrows in H) as well as in isolated clusters remote from the main tumor bulk (I–K). Despite widespread infection within the tumor mass (green fluorescent area in G marked with arrows), no infection was noted in the surrounding brain parenchyma. L–N schematize the general outline of VSV oncolysis in the SCID mouse brain tumor model: After intravenous injection (L), VSVrp30a infection starts at few scattered foci (M) around brain tumor vasculature and spreads to infect the whole tumor (N). Scale bars: A, B, 50 μm; C, 500 μm; D, 300 μm; E, F, H–K, 20 μm; G, 100 μm.
