Figure 5.
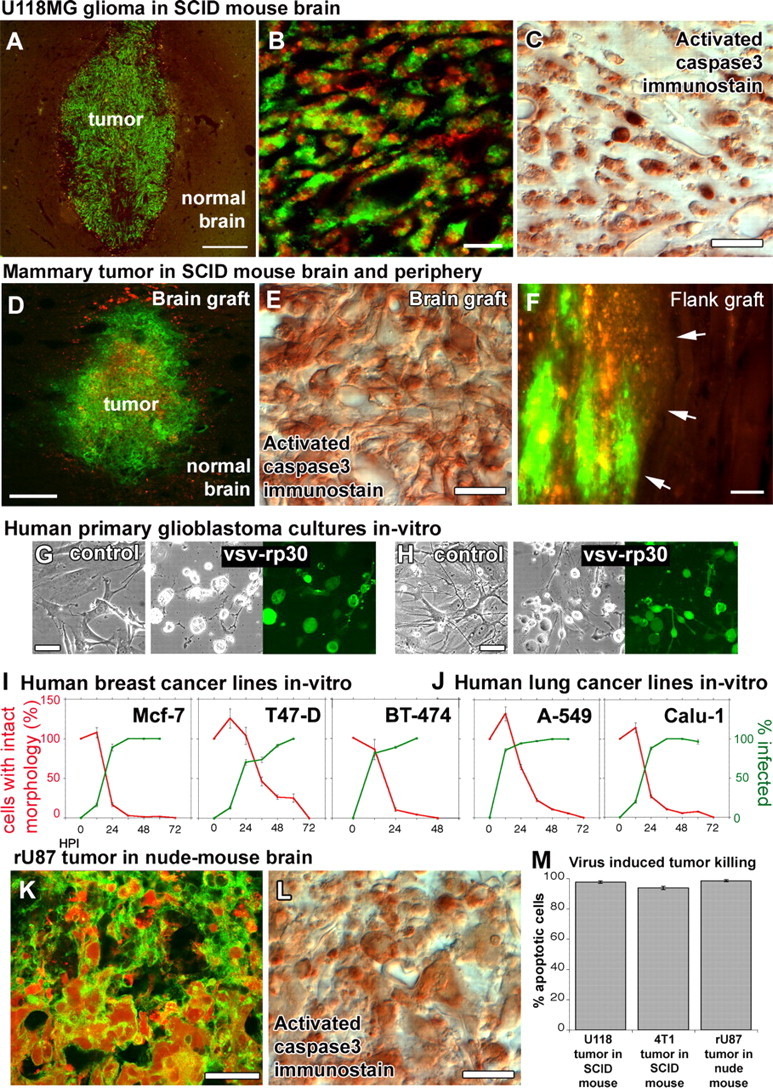
Brain and mammary cancers were targeted in different xenograft models of the brain and the periphery. To exclude the possibility that VSVrp30a tumor targeting might be specific to the rU87 glioma cell line in the SCID mouse model, we tested the virus for its oncolytic activity on different cancer cell lines in vivo and in vitro. Xenografts of rU118 high-grade glioma cells were targeted with VSVrp30a, and the virus spread throughout the whole tumor mass and induced apoptosis within 72 h (A–C). 4T1 mouse mammary cancer cells in the brain (D, E) and in the flank (F) were also effectively and selectively targeted and killed in SCID mice after a single intravenous injection of VSVrp30a (arrows indicate tumor–normal tissue border). Tissue from patients undergoing glioblastoma surgery was cultured and completely infected by VSVrp30a within 36 h (G, H). Three other human breast carcinoma (I) and two human lung carcinoma cell lines (J) were tested in vitro, and all were killed within 48–72 h after the addition of VSVrp30a at a multiplicity of infection of 1. T-cell-deficient nude mice received the same rU87 glioma xenograft and were subjected to the identical virus injection protocol as in the T- and B-cell-deficient SCID mouse model. Comparable with SCID mice, glioma xenografts in the nude mouse brain were efficiently targeted and killed with comparable kinetics (K–M). At 3 d after virus injection, virus-induced apoptosis was observed in the majority of tumor cells. Scale bars: A, D, 300 μm; B, 50 μm; C, 20 μm; E, 30 μm; F, 100 μm; G, H, 30 μm; K, L, 20 μm.
