Figure 3.
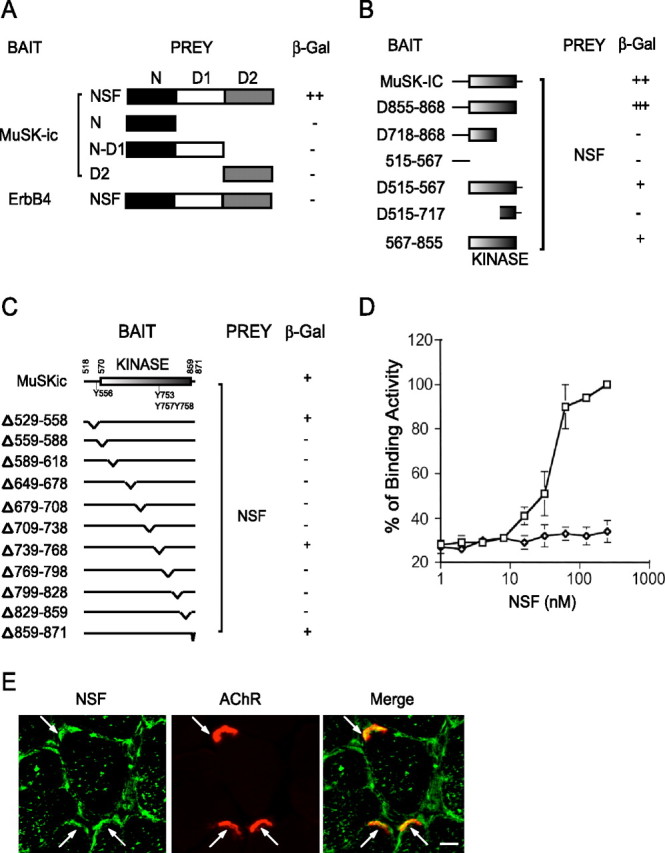
Interaction of MuSK and NSF in yeast. A, Interaction of MuSK and NSF requires the full length of NSF. The domain structure of NSF is shown in the schematic diagram. The original clone isolated from a yeast two-hybrid screen encodes the full-length NSF. The N-terminal domain (amino acids 1–211), N-D1 domain (amino acids 1–495), and D2 domain (amino acids 496–752) were subcloned in pGAD424, fused to C terminus of Gal4AD. These constructs were cotransformed with Gal4BD/MuSKic. Transformed yeast cells were seeded in -Leu-Trp-His plates and scored for β-gal activity. B, Dependence on the MuSK kinase domain for the interaction. Various domains of MuSKic were subcloned in pGBT9 and were cotransformed with Gal4AD/NSF. Interaction was determined as in A. C, NSF interacts with a series of MuSKic mutants with a deletion of 30 aa in the juxtamembrane, kinase, or C-terminal domains. D, Direct interaction between MuSK and NSF. The affinity between MuSK and NSF was determined by ELISA assay. Recombinant MuSK protein was coated in a 96-well plate and incubated with increasing concentrations of GST-NSF fusion proteins or GST alone as negative controls. Bound NSF was detected by monoclonal anti-GST antibody, followed by anti-mouse secondary antibody conjugated to alkaline phosphatase. E, NSF immunoreactivity was detected in AChR clusters as well as in sarcolemma. Denervated muscle sections were incubated with the anti-NSF antibody followed by Alexa 488-conjugated secondary antibody and Alexa 594-conjugated α-BTX. Scale bar, 20 μm. Arrows, NMJ.
