Figure 6.
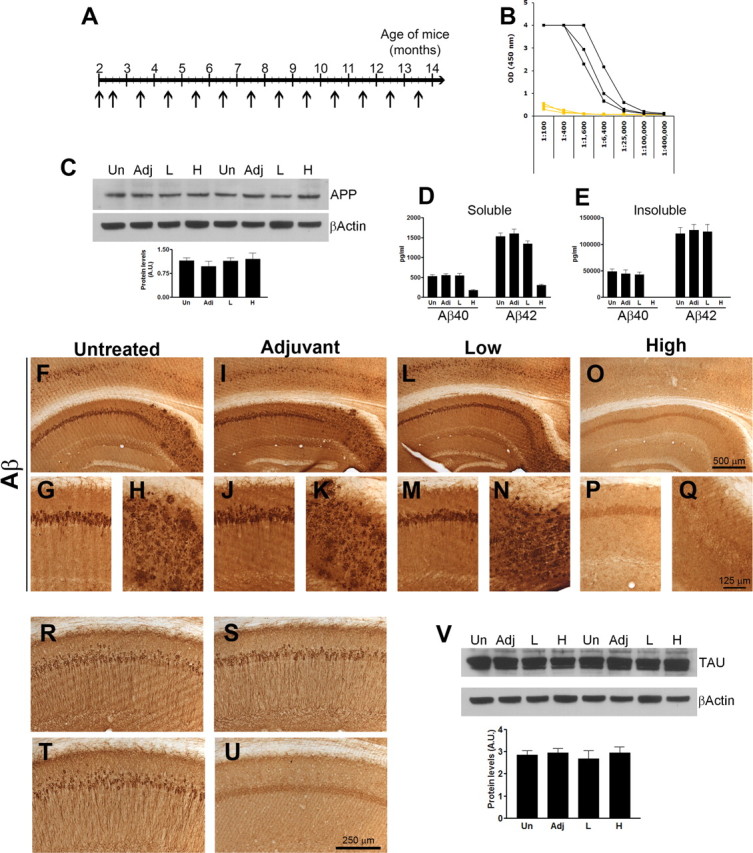
Aβ42 immunization of prepathological 3xTg-AD mice delays the development of tau pathology. To further support the role of Aβ42 in the onset and progression of the tau pathology, we actively immunized 2-month-old 3xTg-AD mice with fibrillar Aβ42 with the goal of preventing Aβ accumulation and determining its effect on the onset and progression of tau pathology. A, Six 2-month-old mice were injected with 100 μg of fAβ42 formulated with 50 μg (initial injection) or 20 μg (subsequent injections) of QuailA adjuvant in a total volume of 100 μl adjusted with PBS. As a control, six mice were injected with adjuvant only and six mice remained untreated. All the mice received a second injection 10 d after the first and monthly thereafter until they were 13.5-month-old. B, Antibody response was only detected in some mice immunized with fAβ42, therefore allowing us to use the low responders as an extra control group. C–E, The steady-state levels of full-length APP were similar among all groups (C), whereas we found a reduction in soluble and insoluble Aβ40 and Aβ42 levels in the brains of the mice with a robust antibody response (D–E). Notably, the insoluble Aβ levels, both 40 and 42, were below detection levels in the immunized mice. F–Q, Whereas the control groups and the low responders show a robust intraneuronal and extracellular Aβ staining, no Aβ deposits were detected in the brains of the mice with a robust antibody response. R–U, Representative photographs indicating that somatodendritic tau levels were drastically reduced in the immunized mice with a robust antibody response (U) compared with untreated (R), adjuvant-treated (S), or low-responder mice (T). V, Western blot analysis indicated that the steady-state levels of tau were similar between all the groups.
