Figure 7.
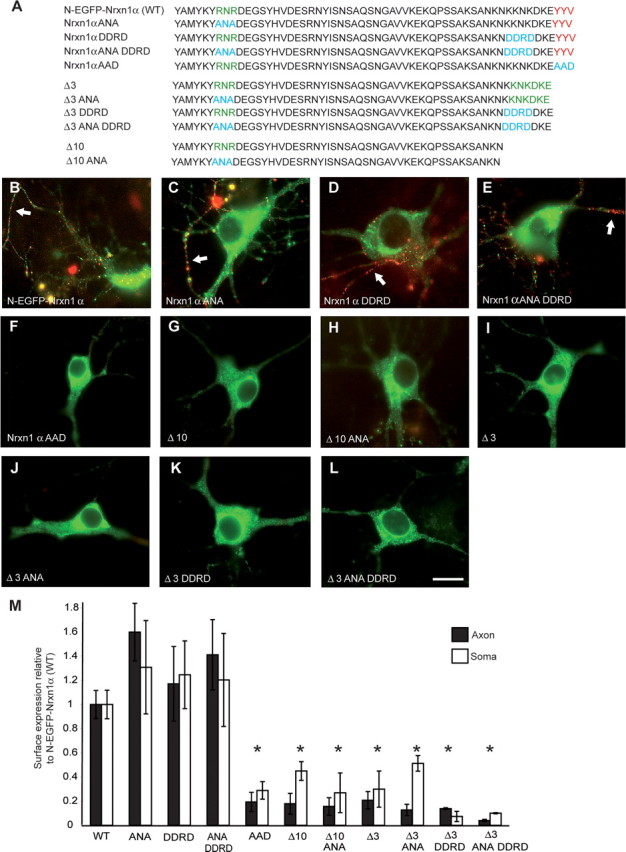
PDZ-binding motif is primarily responsible for targeting of Nrxns. A, Intracellular sequences of wild-type and mutated Nrxn constructs that all contain the identical N-EGFP-Nrxn1α sequences extracellularly (data not shown). Amino acids in red are part of the PDZ-binding motif, green are potential ER retention signals, and blue indicates residues which have been changed. Hippocampal neurons were transfected with either wild-type Nrxn1α (B), Nrxn1α ANA (C), Nrxn1α DDRD (D), Nrxn1α ANA DDRD (E), or Nrxn1α AAD (F). Alternatively, mutants were made from Δ3 already lacking the last three amino acids (I, Δ3; J, Δ3 ANA; K, Δ3 DDRD; or L, Δ3 ANA DDRD), or Δ10 constructs already lacking the last 10 aa (G, Δ10; H, Δ10 ANA). The potential ER retention motif RNR was replaced with ANA, whereas the C-terminal KDKE motif in Δ3 was targeted with the sequence DDRD to disrupt a second potential dilysine motif, KKNK, which partially overlapped with KDKE. All transfected neurons were counterstained with an extracellularly applied antibody to EGFP to detect successful cell-surface insertion (red), and labeling of axonal Nrxn is indicated (white arrows). C–E, Mutating all potential ER retention motifs individually (C, D) or together (E) has no effect on the trafficking of N-EGFP-Nrxn1α. Similarly, mutating these motifs in the Δ3 (J, K, L) or Δ10 deletion constructs (H) does not reverse the mistargeting seen after removing the PDZ-recognition motif (G, I), indicating that the interactions at the motif itself are actively required for correct transport of Nrxns. Consistently, mutating the PDZ-recognition motif YYV to a nonbinding AAD in N-EGFP-Nrxn1α abolished its export in transport vesicles and its insertion into the axonal membrane (F). M, Surface expression of constructs was quantified demonstrating that all mutations affecting the PDZ motif significantly reduced the surface labeling of neuronal axons and soma. *Significantly different to N-EGFP-Nrxn1α at p < 0.05 (n ≥ 3). Scale bar, 20 μm.
