Figure 7.
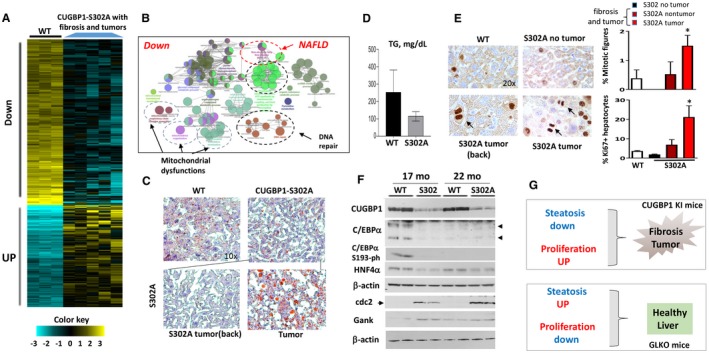
Development of liver fibrosis and tumors in CUGBP1‐S302A mice is associated with down‐regulation of pathways of NAFLD, mitochondrial dysfunctions, and reduction of CUGBP1, C/EBPα, and HNF4α. All the bar graphs represent mean ± SEM. (A) Heat map of RNA‐Seq analysis of livers of five CUGBP1 KI mice and three WT mice. (B) Signaling pathways that are down‐regulated in CUGBP1‐S302A mice that developed fibrosis and tumors. (C) Representative Oil Red O staining of WT mice and CUGBP1 S302A mouse livers. Nontumor and tumor sections of CUGBP1 KI mice are shown. Image magnifications are at 10×. (D) Levels of hepatic TGs were calculated in livers of age‐matched WT and CUGBP1 KI mice with liver tumors. (E) (Left) ki67 staining of livers of WT mice, CUGBP KI mice without tumors, and CUGBP1 KI mice with fibrosis and tumors. Arrows show mitotic figures in the left panel. (Right; bar graphs) Calculations of percentage of mitotic figures and ki6‐positive hepatocytes. * represents P < 0.05 WT Versus CUGBP1 KI mice with fibrosis and tumors. (F) Western blot analysis shows reduction of CUGBP1, C/EBPα, C/EBPα‐S193‐ph, and HNF4α in livers of 17‐ and 22‐month‐old CUGBP1‐S302A mice. cdc2 and Gank were examined as markers of liver proliferation. (G) Hypothesis for the role of steatosis and proliferation in NAFLD and liver diseases. Abbreviations: Down, down‐regulated; mo, months; UP, up‐regulated.
