Figure 1.
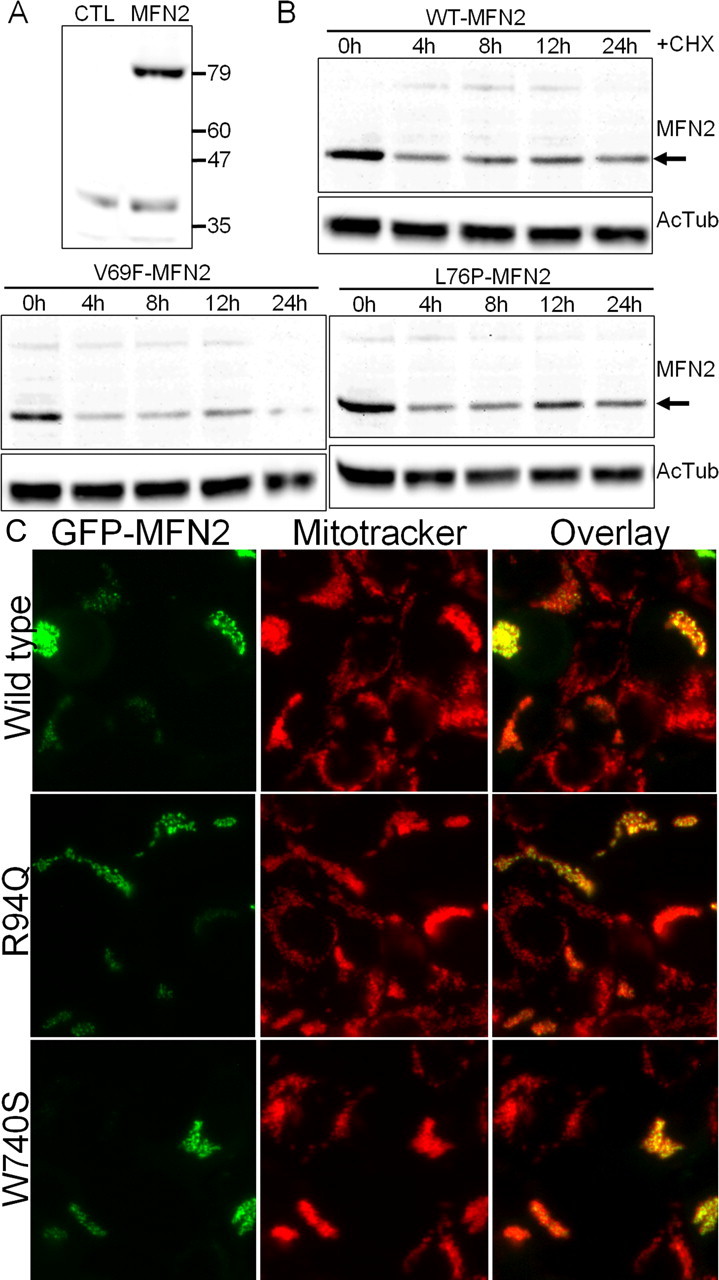
Mutant MFN2 proteins have normal half-lives and are properly localized to mitochondria. A, HEK 293T cells were transfected with an expression vector for GFP (CTL) or wild-type human MFN2 (MFN2), and immunoblotting for MFN2 was performed. Lysate from MFN2-transfected cells showed a single band at ∼80 kDa. Longer exposure did reveal a small amount of endogenous MFN2 present in HEK 293T cells (data not shown). B, HEK 293T cells were transfected with either wild-type (WT-MFN2) or the indicated MFN2 mutant. Cells were lysed at the indicated times after addition of 30 μg/ml cycloheximide (CHX) to inhibit new protein synthesis, and immunoblotting was performed using an anti-MFN2 antibody. Acetylated tubulin (AcTub) immunoblotting is shown on the same samples below as a loading control. Disease mutants ran at identical molecular weight and had a comparable stability to wild-type MFN2. Similar results were obtained for all of the mutants examined in this study (data not shown). C, HEK 293T cells were transfected with wild-type or mutant MFN2 constructs, in which GFP was fused to the N terminus. Cells were treated with Mitotracker Red dye to label mitochondria and imaged with fluorescence microscopy. The overlaid image shows that the GFP-fused MFN2 mutant constructs are properly localized to mitochondria.
