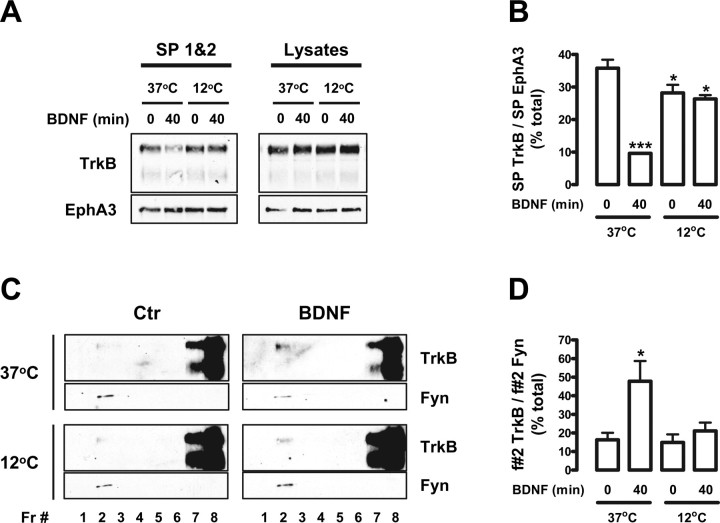
Figure 3.
Blocking TrkB internalization impairs BDNF-induced TrkB recruitment to lipid rafts. A–D, DIV 9 rat cortical neurons were stimulated with BDNF for 40 min, at 37 or 12°C, and used either for cell surface biotinylation (A, B) or for lipid raft fractionation studies (C, D). A, Biotinylated proteins were precipitated with agarose-coupled streptavidin as described in the legend of Figure 2. The combined precipitates from the first two streptavidin precipitations (SP 1&2) were probed with anti-TrkB and anti-EphA3 antibodies. SP3 did not pull down a significant amount of biotinylated TrkB (data not shown). As a loading control, the TrkB and EphA3 levels present in the input lysates are shown on the right. B, Quantification of biotinylated TrkB (SP TrkB) in control and BDNF-treated conditions, at 37 and 12°C, was performed by dividing the intensity of each TrkB-immunoreactive band by the intensity of the corresponding EphA3 band (SP EphA3). Results are expressed as mean percentage of total (the sum of the intensities of all of the bands analyzed) ± SEM for n = 3. C, For lipid raft fractionation studies, the cells were lysed and processed for Optiprep gradient fractionation. Gradient fractions (Fr) were then probed with the mouse anti-TrkB and anti-Fyn antibodies. D, Quantification of lipid raft TrkB in the different conditions was performed by dividing the intensity of each fraction 2 (f#2) TrkB-immunoreactive band by the corresponding Fyn band intensity. Results are expressed as mean percentage of total ± SEM of four independent experiments. The indicated conditions are statistically different from the unstimulated control at 37°C (*p < 0.05; ***p < 0.001).