Figure 10.
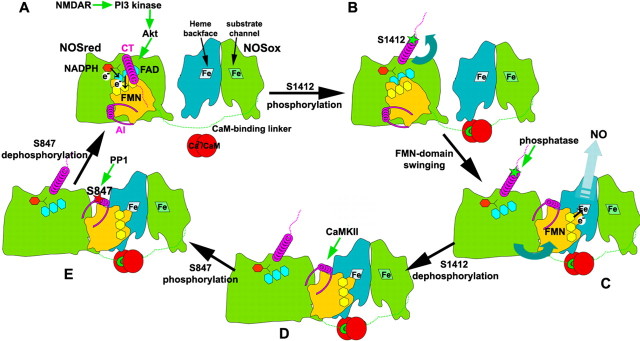
Diagram illustrating the structural mechanism for temporal sequence of nNOS phosphorylations. A, In the presence of NADPH (red hexagon) and the absence of phosphorylations and Ca2+-CaM (red circles), the NOSred dimer (for simplicity, only one monomer is shown) remains in the locked state, and is covalently tethered by the extended CaM-binding linker (green dotted line) to the NOSox dimer [twofold symmetric turquoise and green subunits, with arginine substrate channel and opposing heme (labeled Fe) backface oriented forward on the right and left, respectively]. In the locked state, the N-terminal helical portion (magenta coil) of the C-terminal tail (CT) lies across the interface that the FMN domain (yellow) makes with the remainder of NOSred (green). The FMN (pale yellow) and FAD (cyan) flavin cofactors are buried at this interface, and positioned for the FMN to accept electrons (circled e−) from FAD. With activation of NMDAR, the influx of Ca2+ activates Ca2+-CaM to bind NOS, and to turn on a phosphorylation cascade in which PI3 kinase activates Akt to phosphorylate nNOS at CT S1412. B, In the next step, phosphorylation (green star) of S1412 leads to electrostatic repulsion of the CT from NOSred, which together with Ca2+-CaM binding to the NOS linker, frees the FMN domain from its locked conformation. C, Once released, the FMN domain is free to swing into its proposed electron-donating position, allowing electron delivery to the backface of the NOSox heme belonging to the other polypeptide (turquoise) of dimeric NOS. The open conformation of NOSred exposes CT and the AI helix within the autoinhibitory insert (magenta helix and flanking linkers) of the FMN domain. S1412 is rapidly dephosphorylated by the PP1 phosphatase. D, Meanwhile, Ca2+-CaM influx through activated NMDAR activates CaMKII to phosphorylate AI S847. E, With decreased levels of Ca2+-CaM, S847 is dephosphorylated by PPI, and NOSred returns to the locked conformation (A).