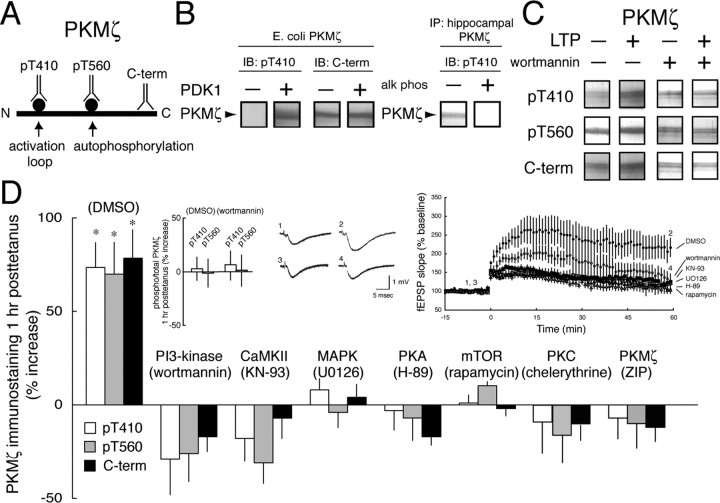
Figure 1.
Synthesis, not phosphorylation of PKMζ is the common target of multiple protein kinases in LTP induction. A, Illustration of the phosphorylation sites and C-terminal (C-term) epitope used for antisera production. B, Specificity of the pT410 antiserum. Left, pT410 antiserum recognizes E. coli-expressed PKMζ after phosphorylation by PDK1; C-terminal antiserum recognizes both phosphorylated and nonphosphorylated PKMζ. Right, Exposure of hippocampal PKMζ to calf intestinal phosphatase eliminates pT410 immunostaining. C, Representative experiment showing PKMζ immunostaining with all three antisera increases after LTP and the blockade of the increases by application of the PI3-kinase inhibitor wortmannin. D, PKMζ immunostaining with all three antisera increases in parallel 1 h after tetanization in the carrier DMSO (0.01%). Asterisks denote p < 0.05. Inhibitors of PI3-kinase, CaMKII, MAPK, PKA, mTOR, PKC, and PKMζ (in 0.01% DMSO) block the synthesis of PKMζ (p > 0.5 between LTP and control slices for each inhibitor; n = 4), but do not affect the relative amounts of phospho-PKMζ and total PKMζ. Insets, Left, Ratios of pT410/C-terminal and pT560/C-terminal immunostaining show no change in the proportion of phosphorylated PKMζ after LTP and blockade of LTP by wortmannin. Middle, Representative fEPSPs for time points shown at the right (DMSO, 1, 2; wortmannin, 3, 4). Right, Time courses of experiments showing LTP in DMSO and blockade of LTP by kinase inhibitors (p > 0.5 between baseline responses and responses 1 h after tetanization for each inhibitor; n = 8).