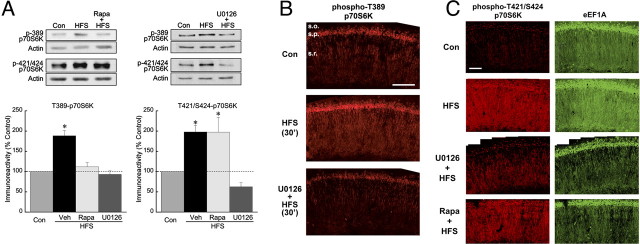
Figure 3.
HFS-induced phosphorylation of the mTOR substrate p70S6K is ERK dependent. A, In CA1 regions from slices frozen 30 min after HFS, p70S6K was hyperphosphorylated at the mTOR-dependent site T389 (left) as well as the putative ERK-dependent site T421/S424. Phosphorylation of both sites was blocked by the MEK inhibitor U0126 (20 μm), whereas 1 μm rapamycin (Rapa) blocked phosphorylation only at T389. The asterisks indicate p ≤ 0.05 versus controls (Con), with all group values of n ≥ 5. B, After HFS (middle panel), immunoreactivity for phospho(T389)-p70S6K was elevated in the apical dendrites and cell bodies of CA1 pyramidal neurons, and diffusely in stratum oriens, compared with vehicle-treated control (Veh) (top panel). Preincubation with 20 μm U0126 (bottom panel) prevented phosphorylation at T389. Scale bar, 150 μm. Images are representative of three experiments. C, HFS increased the immunoreactivity for phospho(T421/S424)-p70S6K (left panels) and for eEF1A (right panels) in dendrites and cell bodies of CA1 pyramidal neurons. The increase in T424/S421-p70S6K phosphorylation was sensitive to U0126 (20 μm) but not to rapamycin (Rapa; 1 μm), whereas both inhibitors prevented the HFS-induced rise in eEF1A expression (bottom two images). These images are representative of three independent experiments. Scale bar, 100 μm.