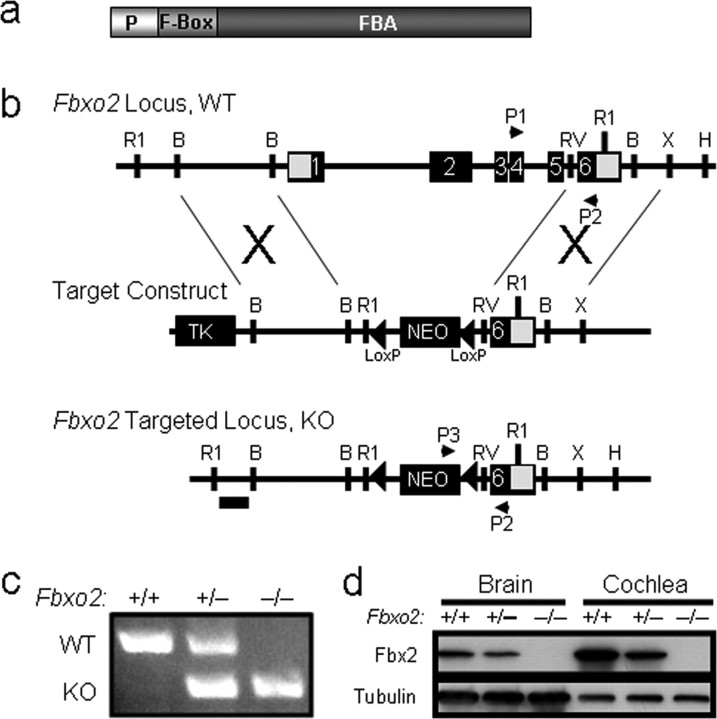
Figure 1.
Deletion of mouse Fbxo2. a, Schematic representation of the Fbx2 protein and its functional domains. The F-box binds Skp1, and the FBA domain binds high-mannose glycoprotein substrates. The PEST domain (P) mediates interactions with CHIP (Nelson et al., 2006) and is necessary for Fbx2 to prevent glycoprotein aggregation in vitro (Yoshida et al., 2007). b, Knock-out strategy for Fbxo2 using homologous recombination. The targeting construct was designed with thymidine kinase (TK) and neomycin (Neo) resistance cassettes. The Neo cassette is flanked with loxP sites (triangles). Successful homologous recombinants lack the first five exons of Fbxo2 and were confirmed by Southern blot analysis using a DNA probe (horizontal bar). Primers P1–P3 were used in PCR genotyping to distinguish WT from KO mice. R1, EcoRI; B, BamHI; RV, EcoRV; X, XhoI. c, Representative PCR genotyping of tail DNA for Fbxo2 in WT (+/+), heterozygotes (+/−), and KO (−/−) mice using primers P1–P3. d, Western blot analysis of Fbx2 expression in brain and cochlea (200 μg of protein in each lane) from wild-type control (Fbxo2+/+), heterozygous (Fbxo2+/−), and knock-out (Fbxo2−/−) mice. Tubulin is shown as a loading control.