Abstract
Endogenous cannabinoids (endocannabinoids) mediate retrograde signals for short- and long-term suppression of transmitter release at synapses of striatal medium spiny (MS) neurons. An endocannabinoid, 2-arachidonoyl-glycerol (2-AG), is synthesized from diacylglycerol (DAG) after membrane depolarization and Gq-coupled receptor activation. To understand 2-AG-mediated retrograde signaling in the striatum, we determined precise subcellular distributions of the synthetic enzyme of 2-AG, DAG lipase-α (DAGLα), and its upstream metabotropic glutamate receptor 5 (mGluR5) and muscarinic acetylcholine receptor 1 (M1). DAGLα, mGluR5, and M1 were all richly distributed on the somatodendritic surface of MS neurons, but their subcellular distributions were different. Although mGluR5 and DAGLα levels were highest in spines and accumulated in the perisynaptic region, M1 level was lowest in spines and was rather excluded from the mGluR5-rich perisynaptic region. These subcellular arrangements suggest that mGluR5 and M1 might differentially affect endocannabinoid-mediated, depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE) in MS neurons. Indeed, mGluR5 activation enhanced both DSI and DSE, whereas M1 activation enhanced DSI only. Importantly, DSI, DSE, and receptor-driven endocannabinoid-mediated suppression were all abolished by the DAG lipase inhibitor tetrahydrolipstatin, indicating 2-AG as the major endocannabinoid mediating retrograde suppression at excitatory and inhibitory synapses of MS neurons. Accordingly, CB1 cannabinoid receptor, the main target of 2-AG, was present at high levels on GABAergic axon terminals of MS neurons and parvalbumin-positive interneurons and at low levels on excitatory corticostriatal afferents. Thus, endocannabinoid signaling molecules are arranged to modulate the excitability of the MS neuron effectively depending on cortical activity and cholinergic tone as measured by mGluR5 and M1 receptors, respectively.
Keywords: endocannabinoid, CB1, 2-arachidonoyl-glycerol (2-AG), diacylglycerol lipase (DAGL), mGluR5, muscarinic acetylcholine receptor M1, immunohistochemistry, striatum, mouse
Introduction
Endogenous cannabinoids (endocannabinoids) are released from postsynaptic neurons, act retrogradely on presynaptic CB1 cannabinoid receptors, and cause short- and long-term suppression of transmitter release. Endocannabinoid-mediated retrograde suppression (ERS) was found originally in the hippocampus (Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001) and cerebellum (Kreitzer and Regehr, 2001; Maejima et al., 2001) and later in other brain regions (Chevaleyre et al., 2006; Hashimotodani et al., 2007). ERS can be triggered by membrane depolarization that elevates intracellular calcium concentration ([Ca2+]i) to a micromolar range (Brenowitz and Regehr, 2003; Maejima et al., 2005), activation of the Gq-protein-coupled metabotropic receptor/phospholipase Cβ (PLCβ) pathway leading to diacylglycerol (DAG) production (Maejima et al., 2001; Kim et al., 2002; Ohno-Shosaku et al., 2003), and their cooperative action (Varma et al., 2001; Ohno-Shosaku et al., 2002; Hashimotodani et al., 2005; Maejima et al., 2005). Of these, the third, cooperative mode appears physiologically the most relevant, because PLCβ serves as a coincidence detector of the two events and enables supralinear DAG production, even when either event is subthreshold for ERS by itself (Hashimotodani et al., 2005; Maejima et al., 2005; Ohno-Shosaku et al., 2005).
The striatum is the main input structure of the basal ganglia subserving motor and cognitive functions (Wilson, 2004). Medium spiny (MS) neurons are the principal neurons, whose activities are sculpted by extrastriatal activities of the sensorimotor cortex, thalamus, and substantia nigra and by GABAergic and cholinergic interneurons (Tepper and Bolam, 2004; Tepper et al., 2004). Excitatory and inhibitory synaptic responses onto MS neurons are sensitive to cannabinoid agonists (Szabo et al., 1998; Gerdeman and Lovinger, 2001; Huang et al., 2001). Moreover, ERS mediates both long-term depression (Gerdeman et al., 2002; Robbe et al., 2002; Ronesi et al., 2004; Kreitzer and Malenka, 2005; Ronesi and Lovinger, 2005; Wang et al., 2006) and short-term suppression at MS neuron synapses (Kreitzer and Malenka, 2005; Freiman et al., 2006; Narushima et al., 2006a,b, 2007; Yin and Lovinger, 2006). Importantly, striatal ERS is facilitated by activation of Gq-protein-coupled receptors, including group I metabotropic glutamate receptors (mGluRs) and M1 muscarinic acetylcholine receptor (mAChR) (Kreitzer and Malenka, 2005; Freiman et al., 2006; Narushima et al., 2006a, 2007).
An endocannabinoid, 2-arachidonoyl-glycerol (2-AG), is synthesized on demand from DAG by sn-1-specific DAG lipase (Mechoulam et al., 1995; Sugiura et al., 1995; Stella et al., 1997; Bisogno et al., 2003). DAG lipase-α (DAGLα) is targeted selectively to somatodendritic compartments and concentrated around spines (Katona et al., 2006; Yoshida et al., 2006), as is the case for group I mGluRs and PLCβ (Baude et al., 1993; Lujan et al., 1996; Nakamura et al., 2004; Mitrano and Smith, 2007). Indeed, DAG lipase inhibitors have been shown to abolish ERS in dopaminergic neurons and Purkinje cells (Melis et al., 2004; Safo and Regehr, 2005; Szabo et al., 2006). These findings together highlight 2-AG as a key mediator of ERS and thus prompted us to determine molecular, anatomical, and physiological bases of 2-AG-mediated ERS in the striatum.
Materials and Methods
Animal.
All experiments were performed according to the guidelines laid down by the animal welfare committees of Hokkaido University and Osaka University and by the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult C57BL/6 mice and null-type gene knock-out mice lacking CB1 (Zimmer et al., 1999) or mGluR5 (stock #003558; The Jackson Laboratory, Bar Harbor, ME) were used in the present study.
Antibody.
We used affinity-purified primary antibodies raised against the following molecules (species immunized); mouse CB1 (rabbit, guinea pig, and goat; see below), mouse DAGLα (rabbit and guinea pig) (Yoshida et al., 2006), rat mGluR5 (rabbit, guinea pig, and goat; see below), mouse mGluR1α (guinea pig) (Tanaka et al., 2000), mouse mAChR M1 (rabbit) (Narushima et al., 2007), dopamine receptors D1R (guinea pig and goat) (Narushima et al., 2006b) and D2R (rabbit) (Narushima et al., 2006b), mouse microtubule-associated protein-2 (MAP2) (goat) (Miura et al., 2006), mouse parvalbumin (PV) (guinea pig and goat) (Nakamura et al., 2004; Miura et al., 2006), nitric oxide synthase (NOS) (rabbit) (Narushima et al., 2007), calretinin [rabbit (#7699/4); Swant, Bellinzona, Switzerland], substance P (SP) [rabbit (AB1566) and guinea pig (AB5892); Millipore, Billerica, MA], Leu-enkephalin (Enk) [rabbit (AB5024); Millipore], mouse vesicular glutamate transporters VGluT1 and VGluT2 (guinea pig and goat) (Miyazaki et al., 2003; Miura et al., 2006), mouse glutamic acid decarboxylase 65/67 (GAD) (rabbit) (Yamada et al., 2001), mouse vesicular GABA transporter (VGAT) (guinea pig and goat) (Miyazaki et al., 2003; Miura et al., 2006), mouse vesicular acetylcholine transporter (VAChT) (rabbit) (Nakamura et al., 2004), mouse plasmalemmal dopamine transporter (DAT) (rabbit, guinea pig, and goat; see below), mouse plasmalemmal serotonin transporter (HTT) (rabbit) (Somogyi et al., 2004), high affinity-choline transporter (CHT) (rabbit) (Narushima et al., 2007), and mouse plasmalemmal norepinephrine transporter (NET) (guinea pig and goat; see below).
For antibody production, cDNA fragments, which are preceded with a BamHI site and encode C-terminal 28 aa of mGluR5 (amino acid residues 1144–1171; GenBank accession number D10891), N-terminal 60 aa of DAT (1–60; GenBank accession number BC054119), or N-terminal 56 aa of NET (1–56; GenBank accession number AY188506), were obtained by PCR using single-stranded mouse brain cDNA library. After the TA cloning using a pGEM-T Easy Vector System I kit (Promega, Madison, WI), cDNA fragments were sequenced and excised by BamHI and EcoRI. These fragments were subcloned into pGEX4T-2 plasmid (GE Healthcare, Piscataway, NJ) for expression of glutathione S-transferase (GST) fusion proteins. GST fusion proteins were purified using glutathione–Sepharose 4B (GE Healthcare), according to the manufacturer's instructions. GST fusion proteins were emulsified with Freund's complete or incomplete adjuvant (DIFCO, Detroit, MI) and immunized subcutaneously to New Zealand White rabbits, Hartley guinea pigs, and goats at intervals of 2 weeks. After the sixth injection, Igs specific to antigens were affinity-purified using GST-free peptides coupled to CNBr-activated Sepharose 4B (GE Healthcare). GST-free peptides were prepared by in-column thrombin digestion of GST fusion proteins bound to glutathione-Sepharose 4B media. In the present study, CB1 antibody was raised newly against the same antigen as used previously (Fukudome et al., 2004). The specificity of antibody and immunohistochemistry for DAGLα was confirmed by the disappearance of characteristic immunolabeling when using DAGLα antibody preabsorbed with the antigen, whereas that for mGluR5 and CB1 was done by the lack of characteristic immunolabelings in the brains of knock-out mice (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). The specificity of DAT and NET antibodies was confirmed in Western blots by selective detection of protein band at 60–65 or 45 kDa, respectively, and by selective neuronal labeling in the substantia nigra pars compacta or in the locus ceruleus, respectively (data not shown).
Immunohistochemistry.
Under deep pentobarbital anesthesia (100 mg/kg body weight, i.p.), mice were fixed transcardially with 4% paraformaldehyde in 0.1 m sodium phosphate buffer (PB), pH 7.2, for light microscopy or 4% paraformaldehyde/0.1% glutaraldehyde in PB for electron microscopy. Thick sections (50 μm in thickness) were prepared by microslicer (VT1000S; Leica, Nussloch, Germany) and subjected to the free-floating method, whereas semithin cryosections (200 nm) were prepared by ultracryomicrotome (EM-FCS; Leica) and mounted on silane-coated glass slides.
All immunohistochemical incubations were done at room temperature. For immunofluorescence, microslicer sections and semithin cryosections were incubated successively with 10% normal donkey serum for 30 min, mixture of primary antibodies overnight (1 μg/ml), and mixture of Alexa 488-, indocarbocyanine (Cy3)-, and indodicarbocyanine (Cy5)-labeled species-specific secondary antibodies for 2 h at a dilution of 1:200 (Invitrogen, Eugene, OR; Jackson ImmunoResearch, West Grove, PA). Images were taken with a confocal laser-scanning microscope (FV1000; Olympus Optical, Tokyo, Japan).
For immunoperoxidase electron microscopy, microslicer sections were incubated successively with 10% normal donkey serum, primary antibodies (1 μg/ml), biotinylated secondary antibody, and streptavidin–peroxidase complex as above. Immunoreaction was visualized with 3,3′-diaminobenzidine. For preembedding immunogold electron microscopy, microslicer sections were dipped in 5% bovine serum albumin (BSA)/0.02% saponin/PBS for 30 min and incubated overnight with primary antibodies diluted with 1% BSA/0.004% saponin/PBS and then with secondary antibodies linked to 1.4 nm gold particles (Nanogold; Nanoprobes, Stony Brook, NY) for 2 h. Immunogolds were intensified with a silver enhancement kit (HQ silver; Nanoprobes). When combining the two methods for double immunoelectron microscopy, we executed both orders and confirmed essentially the same results. However, pictures shown in Figure 9A–D were taken from the specimens that had been subjected first to immunoperoxidase and then to immunogold, because this order prevented silver-enhanced immunogold particles from reductions in size and electron density. Sections labeled by immunoperoxidase and silver-enhanced immunogold were treated with 1% osmium tetroxide for 15 min, stained in block with 2% uranyl acetate for 20 min, dehydrated, and embedded in Epon 812. Photographs were taken with an H-7100 electron microscope (Hitachi, Tokyo, Japan). For quantitative analysis, cell membrane-attached immunogold particles were counted on electron micrographs and analyzed using IPLab software (Nippon Roper, Tokyo, Japan). The mean number of membrane-attached gold particles/1 μm of plasma membrane was counted for each neuronal compartment (dendritic spine, dendritic shaft, soma, and axon). Statistical significance was assessed by Student's t test.
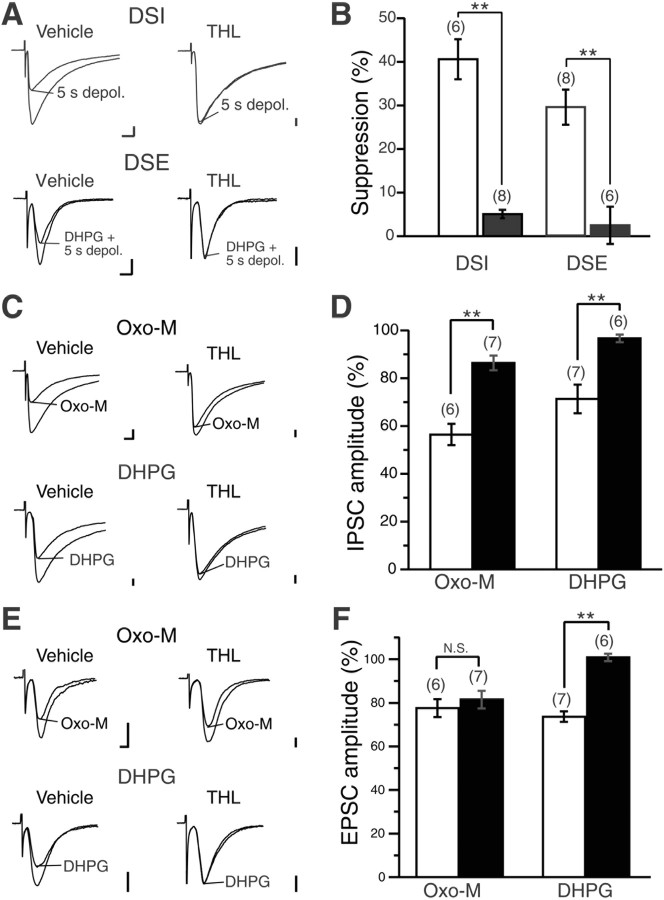
Figure 9.
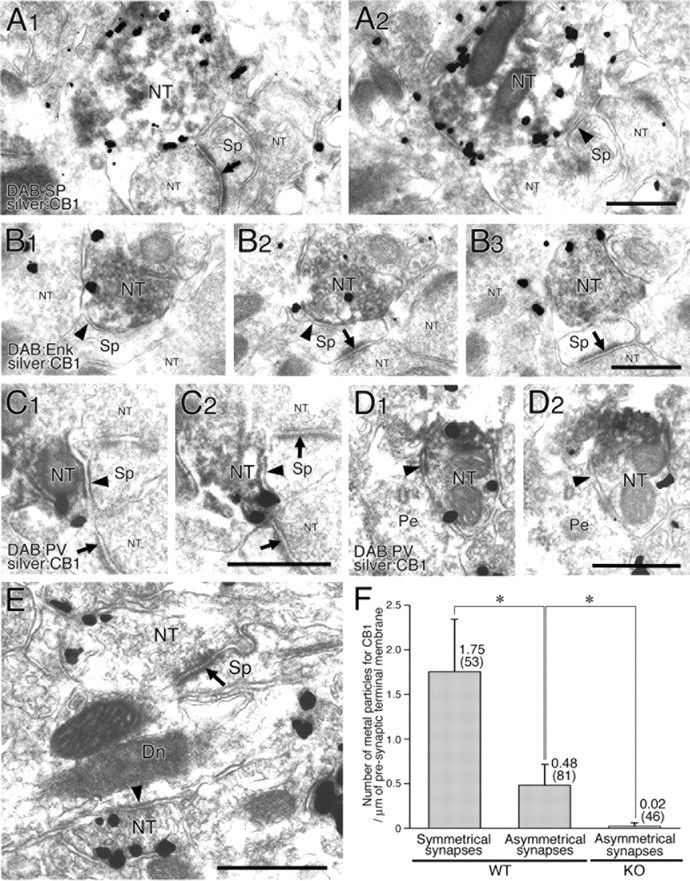
Immunoelectron microscopy demonstrating dense CB1 localization in axons and terminals of MS neurons and PV interneurons. A1–D2, Double labeling by preembedding silver-enhanced immunogold for CB1 and immunoperoxidase for neuronal markers (A1, A2, SP; B1–B3, Enk; C1–D2, PV). Note the dense CB1 labeling on the cell membrane of SP-, Enk-, or PV-positive nerve terminals (NT in larger font), all forming symmetrical synapses (arrowheads) onto dendritic spines (Sp) or perikarya (Pe). Arrows indicate asymmetrical synapses, whose presynaptic nerve terminals are rarely labeled by double-labeling experiment, because of lower sensitivity than single immunogold labeling. Two or three consecutive images from serial sections show reproducible immunogold labeling on the terminal membrane. E, Single immunogold labeling for CB1. Low but significant labeling can be detected on the terminal membrane (top left portion; NT) forming an asymmetrical synapse (arrow) with an MS neuron spine. See heavy labeling on a nerve terminal forming a symmetrical synapse (arrowhead) with a dendritic shaft (Dn). F, The mean number of metal particles per 1 μm of presynaptic terminal membrane. CB1 labeling is 3.6 times higher at symmetrical synapses than at asymmetrical synapses in wild-type (WT) control mice. The background level is determined from labeling density at asymmetrical synapses of CB1 knock-out (KO) mice. Numbers in parentheses indicate the number of synaptic profiles examined. The total length of measured cell membrane is 101.7 μm for symmetrical synapses in wild-type mice, 140.3 μm for asymmetrical synapses in wild-type mice, and 81.2 μm for asymmetrical synapses in CB1 knock-out mice. Error bars indicate the mean ± SD. *p < 0.01. Scale bars, 500 nm.
Electrophysiology.
Coronal brain slices containing the cortex and the striatum (300 μm in thickness) were prepared from C57BL/6 mice aged to postnatal days 15–22 as described previously (Narushima et al., 2006a,b). Mice were decapitated under deep halothane anesthesia, and the brains were cooled in ice-cold, modified external solution containing the following (in mm): 120 choline-Cl, 2 KCl, 8 MgCl2, 28 NaHCO3, 1.25 NaH2PO4, and 20 glucose bubbling with 95% O2 and 5% CO2 (Narushima et al., 2006a,b). Slices were cut with Leica VT1000S slicer. For recovery, slices were incubated for at least 1 h in normal bathing solution composed of the following (in mm): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, and 20 glucose, pH 7.4, which was bubbled continuously with a mixture of 95% O2 and 5% CO2 at room temperature. Whole-cell recordings were made from MS neurons in dorsolateral region of the striatum, using an upright microscope (BX50WI; Olympus Optical) equipped with infrared-CCD camera system (Hamamatsu Photonics, Hamamatsu, Japan) at 32°C. MS neurons were identified visually by their medium-sized, spherical somata. Resistance of the patch pipette was 3–5 MΩ when filled with the standard intracellular solution composed of the following (in mm): 50 CsCl, 90 Cs-gluconate, 10 HEPES, 1 EGTA, 0.1 CaCl2, 4.6 MgCl2, 4 Na-ATP, and 0.4 Na-GTP, pH 7.2, adjusted with CsOH. The pipette access resistance was compensated by 50–70%. MS neurons were usually held at a membrane potential of −80 mV. For recording EPSCs, the bath solution was supplemented with 20 μm (−)-bicuculline methochloride and 5 μm (R)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic [(R)-CPP; Tocris Bioscience, Bristol, UK]. Cortical EPSCs were evoked by stimulating white matter with bipolar platinum electrodes. For recording IPSCs, the bath solution was supplemented with 10 μm 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo quinoxaline-7-sulfonamide (NBQX; Tocris Bioscience) and 5 μm (R)-CPP. Glass pipettes filled with normal saline were used to stimulate putative GABAergic fibers. Membrane currents were recorded with an EPC8 or EPC9 amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany). The PULSE software (HEKA Elektronik) was used for stimulation and data acquisition. The signals were filtered at 3 kHz and digitized at 20 kHz.
For testing effects of chemicals, two successive stimuli (duration, 0.1 ms; amplitude, 0–90 V) with an interstimulus interval of 50 ms were applied every 20 s. All drugs were bath applied. Effects of drugs were estimated as the percentage of the mean amplitudes of five consecutive IPSCs during drug application relative to that before application. In the experiments with tetrahydrolipstatin (THL), striatal slices were incubated with normal saline supplemented with 20 μm THL and 0.2 mg/ml BSA or BSA alone, for at least 1 h. Then the slice was moved to the recording chamber and was also superfused continuously with the external solution containing 10 μm THL during recording. Slices incubated with BSA alone were used as controls. Oxotremorine-M (oxo-M), (RS)-3,5-dihydroxyphenylglycine [(RS)-3,5-DHPG; DHPG] and (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate (WIN 55,212-2) were purchased from Tocris Bioscience. BSA and THL were from Sigma-Aldrich (St. Louis, MO).
To induce depolarization-induced suppression of inhibition (DSI) or depolarization-induced suppression of excitation (DSE), a depolarizing pulse (0.1, 1, or 5 s duration from −80 to 0 mV) was applied to MS neurons. The magnitude of suppression was calculated as the percentage of reduction in the mean of three consecutive response amplitudes after depolarization relative to that of 10 consecutive response amplitudes just before depolarization. To quantify enhancement of DSI or DSE by some experimental manipulation, ΔDSI or ΔDSE was calculated by subtracting the DSI/DSE magnitude in the control condition from that after the experimental manipulation.
Averaged data from different experiments are presented as mean ± SEM. Statistical significance was assessed by Mann–Whitney U test (for comparison of two independent samples) or Wilcoxon signed rank test (for paired comparison of the same sample).
Results
In the present study, 2-AG-mediated retrograde signaling was studied in the mouse striatum. All anatomical and physiological experiments were performed in the dorsolateral portion of the striatum, which is known to receive corticostriatal afferents from the sensorimotor cortex (Deniau et al., 1996; Ramanathan et al., 2002) and has the highest CB1 expression level (Herkenham et al., 1991; Matsuda et al., 1993; Egertova and Elphick, 2000; Hohmann and Herkenham, 2000; Matyas et al., 2006).
DAGLα is widely expressed on the somatodendritic surface of MS neurons with a distal-to-proximal gradient
To unravel the precise production site of 2-AG, we first examined the immunohistochemical localization of its synthetic enzyme DAGLα (Figs. 1, 2). DAGLα was distributed moderately throughout the striatum with higher distribution in the dorsolateral portion than in the remaining portions (supplemental Fig. S1B,C, available at www.jneurosci.org as supplemental material). Immunofluorescence at high magnifications visualized DAGLα as tiny puncta distributed on the surface of D1R- and D2R-positive bushy elements (Fig. 1A1,A2). The immunolabeled elements were judged mostly, if not all, to be somatodendritic neuronal elements, because DAGLα was preferentially distributed on the surface of MAP2-positive dendrites (Fig. 1B1–C2, arrows) and perikarya (Fig. 1C1,C2, arrowheads). Notably, the intensity of individual DAGLα puncta was apparently higher on D1R-positive elements (Fig. 1A1,A2, asterisks) than on D2R-positive ones (Fig. 1A1,A2, pound signs). In contrast, DAGLα showed no apparent overlap with glutamatergic terminal marker VGluT1 or inhibitory terminal marker VGAT but was distributed among these immunostained terminals (Fig. 1D1,D2). These results suggest that DAGLα is present as molecular clusters, which are distributed on the somatodendritic surface of the D1R- and D2R-positive MS neurons and are adjacent to both excitatory and inhibitory terminals. Furthermore, although we found no overlapping of DAGLα with the main target of 2-AG, the CB1 cannabinoid receptor, this lipase was positioned very close to CB1 (Fig. 1E1,E2).
Figure 1.
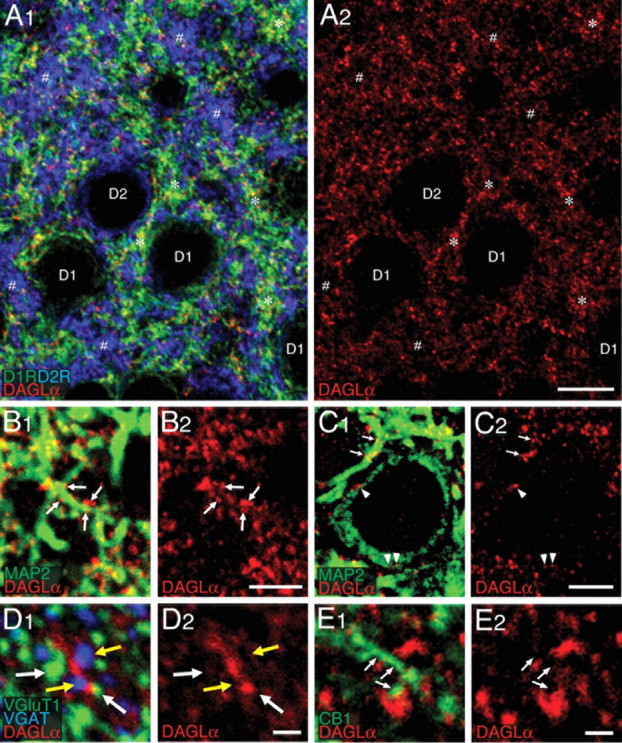
DAGLα is expressed on somatodendritic elements of D1R-positive and D2R-positive medium spiny neurons. Throughout this Figure, DAGLα is pseudocolored in red. A1, A2, Triple immunofluorescence for DAGLα, D1R (green), and D2R (blue). Note a punctate pattern of DAGLα immunolabeling, which is higher on D1R-positive elements (*) than on D2R-positive elements (#). B1– C2, Double immunofluorescence for DAGLα and MAP2 (green). DAGLα is distributed on the surface of MAP2-positive dendrites (arrows) and perikarya (arrowheads). From thin perikaryal rim and round nucleus, the neuron in C1 and C2 is a putative MS neuron. D1, D2, Triple immunofluorescence for DAGLα, VGluT1 (green; white arrows), and VGAT (blue; yellow arrows). E1, E2, Double immunofluorescence for DAGLα and CB1 (green; arrows). Scale bars: (in A2) A1, A2, 10 μm; (in B2, C2) B1–C2, 5 μm; (in D2, E2) D1–E2, 1 μm.
Figure 2.
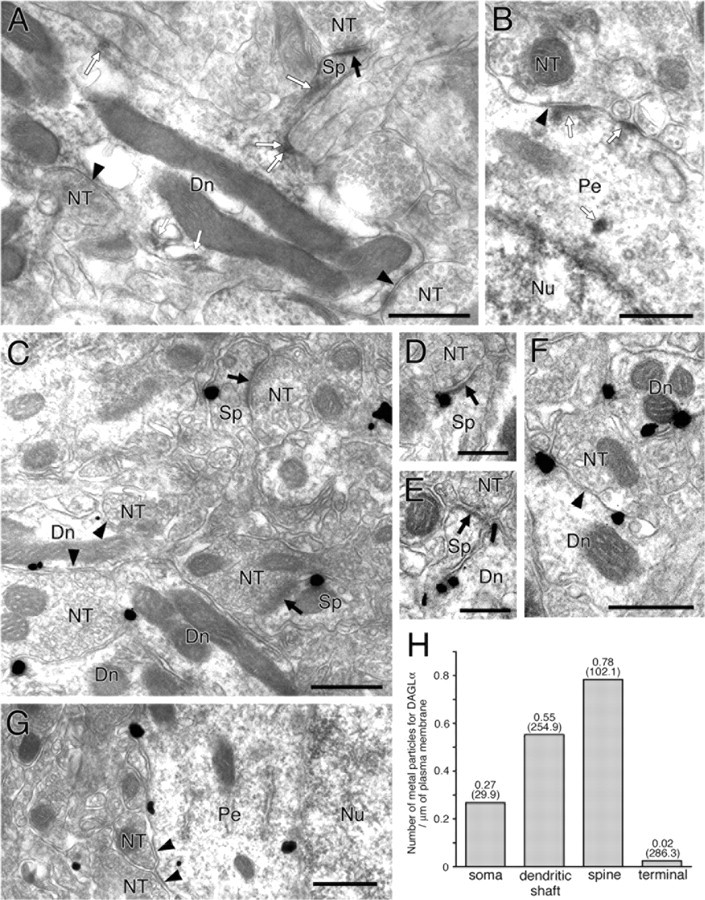
Immunoelectron microscopy demonstrating somatodendritic expression of DAGLα in spines, dendritic shafts, and somata of medium spiny neurons. Black arrows and arrowheads indicate asymmetrical or symmetrical synapses, respectively. A, B, Immunoperoxidase showing spotty labeling for DAGLα (open arrows) in spines (Sp), shaft dendrites (Dn), and perikarya (Pe) of presumed MS neurons. C–G, Silver-enhanced immunogold showing preferential cell surface distribution of DAGLα in spine head and neck, shaft dendrites, and somata of presumed MS neurons. H, The mean number of metal particles for DAGLα per 1 μm of the cell membrane. From very low density in nerve terminals, DAGLα is judged to be distributed selectively in somatodendritic compartments. Numbers in parentheses indicate the total length of measured cell membrane. NT, Nerve terminal; Nu, nucleus. Scale bars: A–C, F, G, 500 nm; D, E, 250 nm.
This observation was verified and further characterized by immunoelectron microscopy (Fig. 2). Immunoperoxidase reaction products for DAGLα were observed as spotty deposition in spines, shafts of spiny dendrites, and somata with thin perikarya and round nuclei (Fig. 2A,B, open arrows). Using the silver-enhanced immunogold technique, metal particles representing DAGLα were detected on the cell membrane or around cytoplasmic vesicles in spines, dendritic shafts, and somata (Fig. 2C–G). On these somatodendritic elements, metal particles were found preferentially on the extrasynaptic membrane and were very rare on the postsynaptic membrane of asymmetrical (Fig. 2C–E, arrows) and symmetrical (Fig. 2C,F,G, arrowheads) synapses. To quantify more precisely this distribution pattern, the density of immunogold particles attached to the cell membrane was measured at the electron microscopic level. The density of DAGLα was highest in spines and decreased gradually toward somata (Fig. 2H). The density on the somatic membrane was three times lower than on the spine membrane but was even much higher than on nerve terminals. Therefore, DAGLα is distributed widely on extrasynaptic somatodendritic surface of MS neurons in the order of spine > dendritic shaft > soma.
Subcellular distribution of mGluR5 and DAGLα are similar to each other but different from mAChR M1
Previous studies have shown that group I mGluR activation facilitates both DSI and DSE in MS neurons (Kreitzer and Malenka, 2005; Freiman et al., 2006; Narushima et al., 2006a). Our finding on the abundant DAGLα localization in spines prompted us to scrutinize the precise distribution of these receptors (mGluR1 and mGluR5) in the striatum in relation to DAGLα localization. In the forebrain, the striatum has the lowest level of mGluR1α immunostaining (supplemental Fig. S2A, available at www.jneurosci.org as supplemental material) but the highest level of mGluR5 immunostaining (supplemental Fig. S1E,F, available at www.jneurosci.org as supplemental material). Experiments with cell-type-specific neuronal markers revealed that this is attributable to the very low or to the very high expression of mGluR1α (supplemental Fig. S2B–E, available at www.jneurosci.org as supplemental material) or mGluR5 (see below), respectively, in MS neurons. Thus, mGluR5 is the major group I mGluR in MS neurons.
Similarly to DAGLα, mGluR5 was detected in both D1R-positive and D2R-positive elements (Fig. 3A1,A2), and was preferentially distributed on MAP2-positive dendrites (Fig. 3B1–C2, arrows) and perikarya (Fig. 3C1,C2, arrowheads). Different from DAGLα, however, no appreciable differences were discerned in the intensity of mGluR5 immunoreactivity between D1R-positive and D2R-positive elements (Fig. 3A1,A2). By silver-enhanced immunogold labeling, dense distribution of mGluR5 was demonstrated on the surface of spines, shafts of spiny dendrites, and somata of MS neurons (Fig. 3D–G). Precise quantification of high-resolution immunogold labeling revealed a decreasing density gradient in the order of spine > dendritic shaft > soma (Fig. 3H). The density in somata was twice as low as in spines, but was still much higher compared with nerve terminals. These results indicate that mGluR5 displays subcellular distribution similar to DAGLα in MS neurons.
Figure 3.
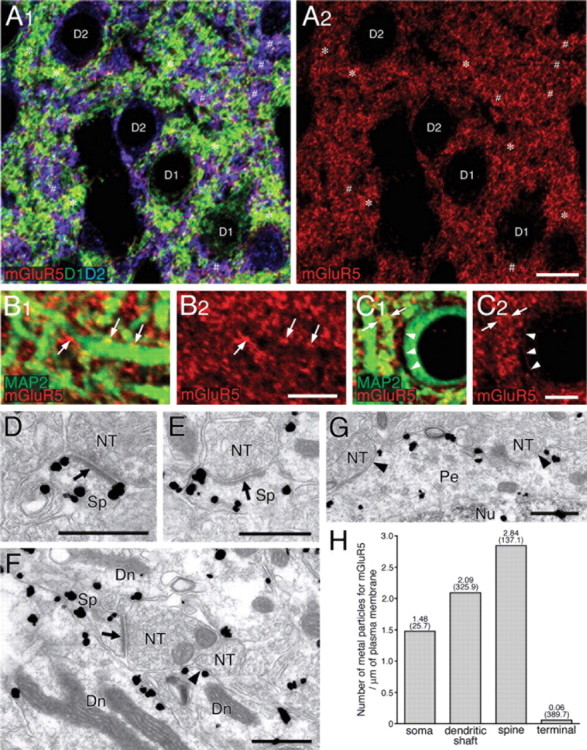
mGluR5 is distributed on somatodendritic elements of D1R-positive and D2R-positive medium spiny neurons. A1, A2, Triple immunofluorescence for mGluR5 (red), D1R (green), and D2R (blue). Note a punctate or reticular pattern of mGluR5 immunolabeling, which is detected on D1R-positive (*) and D2R-positive (#) elements in the neuropil. B1–C2, Double immunofluorescence for mGluR5 (red) and MAP2 (green). In addition to dense mGluR5 labeling in the neuropil, low to moderate labeling can be detected on the surface of shaft dendrites (arrows) and somata (arrowheads) of presumed MS neurons. D–G, Silver-enhanced immunogold showing preferential cell surface distribution of mGluR5 in spines (Sp), dendritic shafts (Dn), and perikarya (Pe) of presumed MS neurons. Arrows and arrowheads indicate asymmetrical and symmetrical synapses, respectively. NT, Nerve terminal; Nu, nucleus. H, The mean number of metal particles for mGluR5 per 1 μm of the cell membrane. From very low density in terminals, mGluR5 is judged to be specific to somatodendritic compartments, similarly to DAGLα. Numbers in parentheses indicate the total length of measured cell membrane. Scale bars: A2, 10 μm; B2, C2, 2 μm; D–G, 500 nm.
M1 muscarinic receptor is another Gq-coupled receptor expressed abundantly in MS neurons (Yan et al., 2001; Narushima et al., 2007), and its activation significantly enhances DSI (Narushima et al., 2007). We have recently shown that M1 also displays widespread somatodendritic distribution in MS neurons, but its density is lower in spines than in dendritic shafts and somata (Narushima et al., 2007). In the present study, we compared their fine distribution on the spine head of MS neurons (Fig. 4). Wide extrasynaptic distribution in spines was common to both mGluR5 and M1. However, mGluR5 tended to accumulate at the border between the postsynaptic density and the extrasynaptic membrane (0–40 nm from the edge of the synaptic junction) (Fig. 4A), whereas M1 was rather low at this mGluR5-rich perisynaptic region (Fig. 4B). Therefore, mGluR5 is enriched in spines of MS neurons, particularly at the perisynaptic region, whereas M1 is relatively sparse in spines and appears to be excluded from this perisynaptic region.
Figure 4.
A, B, Histograms showing tangential distribution of cell membrane-associated immunogold particles for mGluR5 (A) and M1 (B) on the spine head of medium spiny neurons. Immunoreaction was performed by preembedding silver-enhanced immunogold. The edge of postsynaptic density is defined as 0 with the synaptic and extrasynaptic side to the left and right, respectively. Note that the very perisynaptic region (0–40 nm bin, black columns) is provided with the highest labeling for mGluR5 but the lowest labeling for M1 on the spine head. The total number of immunogold particles analyzed is 82 particles for mGluR5 and 69 particles for M1. The total length of measured cell membrane is 36.0 μm for mGluR5 and 46.5 μm for M1.
2-AG is the major endocannabinoid for striatal DSI and DSE, and its release is facilitated by mGluR and/or mAChR activation
Strong depolarization of MS neurons triggers endocannabinoid-mediated suppression of striatal excitatory (Narushima et al., 2006a) and inhibitory (Narushima et al., 2006b) transmissions, and activation of M1 mAChRs (Narushima et al., 2007) or group I mGluRs (Freiman et al., 2006; Narushima et al., 2006b) enhances this suppression. Considering that 2-AG is synthesized from phosphatidylinositol by PLCβ and DAG lipase in an activity-dependent manner (Stella et al., 1997; Piomelli, 2003), 2-AG is likely to be synthesized after depolarization combined with activation of these Gq-coupled receptors and to act as an endocannabinoid at MS neuron synapses. To pursue this possibility, we examined the effect of the DAG lipase inhibitor tetrahydrolipstatin (THL) (Bisogno et al., 2003) on endocannabinoid-mediated modulations.
First, we tested THL on DSE or DSI. Bath application of THL (10 μm) after preincubation of slices with THL (20 μm) significantly blocked DSI induced by 5 s depolarization. The magnitude of DSI was 40.6 ± 4.6% in vehicle solution (n = 6), whereas it was 5.1 ± 1.0% in the presence of THL (n = 8, p = 0.0019) (Fig. 5A,B). Because Palomaki et al. (2007) have reported very recently that THL specifically antagonizes endocannabinoid-dependent CB1 receptor activity, we tested whether THL has any effect on CB1-induced suppression of IPSCs in MS neurons (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). After we confirmed that the THL treatment abolished DSI, bath application of the cannabinoid agonist WIN 55,212-2 (1 μm) reduced the IPSC amplitude to 35.9 ± 4.7% (n = 5), which was not distinguishable from the suppression by WIN 55,212-2 in normal bathing solution (32.9 ± 3.6%; n = 11) (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). Therefore, THL did not affect CB1 receptor function in our experimental condition.
Figure 5.
DAGL inhibitor prevents endocannabinoid-mediated retrograde signaling in the striatum. A, Sample traces of IPSC (top) and EPSC (bottom) showing the suppression induced by 5 s depolarization (depol.) in vehicle solution (left) and in the presence of THL (right). For EPSC, 50 μm DHPG was supplemented to induce DSE. B, Summary bar graph showing average magnitudes of DSI and DSE after 5 s depolarization in vehicle solution (open columns) and in the presence of THL (filled columns). C, Sample traces showing the effect of 1 μm oxo-M or 50 μm DHPG on IPSCs recorded from MS neurons in the BSA-containing vehicle solution (vehicle) and in the presence of THL (THL). D, Summary bar graph showing the suppression of IPSCs by oxo-M or DHPG. Data are expressed as a percentage of IPSC amplitudes in oxo-M or DHPG relative to the values before oxo-M or DHPG application in the vehicle solution (open columns) and in the presence of THL (filled columns). E, Sample traces showing the effect of oxo-M and DHPG on EPSCs. F, Summary bar graph showing the effect of oxo-M and DHPG on EPSCs. N.S., Not significant. Data are illustrated similarly to D. Calibration: 100 pA, 5 ms. **p < 0.01.
To induce discernible DSE of corticostriatal EPSCs, we combined 5 s depolarization of MS neurons with 50 μm DHPG, a group I mGluR agonist, because striatal DSE is observed only when long depolarization is combined with group I mGluR activation (Narushima et al., 2006a). This DHPG-enhanced DSE was also blocked by THL (magnitude of DSE, 29.5 ± 4.0% in vehicle solution, n = 8; 2.4 ± 4.3% in the presence of THL, n = 6, p = 0.0019) (Fig. 5A,B). Then, we examined THL on endocannabinoid-mediated synaptic suppression triggered by Gq-coupled receptor activation. For IPSCs, THL effectively blocked both DHPG-induced and the mAChR agonist oxo-M-induced suppressions (Fig. 5C,D). The averaged amplitude after DHPG or oxo-M application was 71.4 ± 6.0% (n = 7) or 56.5 ± 4.4% (n = 6) in vehicle-containing control solution, whereas the amplitude was 96.7 ± 1.7% (n = 6; p = 0.0027) or 91.2 ± 4.4% (n = 7; p = 0.0027) in THL. DHPG-induced suppression of corticostriatal EPSCs was also blocked by THL (averaged amplitude after DHPG application, 73.7 ± 2.4% in vehicle, n = 7; 100.8 ± 1.7% in THL, n = 6, p = 0.0027) (Fig. 5E,F). In contrast, THL had little effect on oxo-M-induced suppression of EPSCs (averaged amplitude after oxo-M application; 77.5 ± 4.1% in vehicle, n = 6; 81.5 ± 4.1% in THL, n = 7, p = 0.57). It has been shown that oxo-M-induced suppression of corticostriatal EPSC is mediated by presynaptic mAChR and is independent of CB1 (Narushima et al., 2006a) (Fig. 5E,F). These results clearly indicate that postsynaptic depolarization, Gq-coupled receptor activation, and combined depolarization and Gq-coupled receptor activation all induce DAG lipase-mediated synthesis of 2-AG, which results in suppression of transmitter release at both GABAergic and glutamatergic synapses of MS neurons.
DSE exhibits lower sensitivity to DHPG than DSI for enhancement
The different relative abundance of mGluR5 and M1 on the somatodendritic surface and at the perisynaptic region suggests that these receptors may exhibit differential effects on 2-AG-mediated suppression of glutamatergic and GABAergic synaptic transmission. Indeed, application of oxo-M can enhance DSI but not DSE (Narushima et al., 2006a) (Fig. 5F). However, DSE and DSI are both enhanced by DHPG (Freiman et al., 2006; Narushima et al., 2006a) (Fig. 5C–F), but their sensitivities to DHPG have not been compared quantitatively.
To address the aforementioned issue, the dose dependency of DSE/DSI enhancement on DHPG was compared by application of DHPG at concentration of 1, 5, or 25 μm, which treatment per se had little effect on the amplitude of IPSC or EPSC in MS neurons. DSI after 1 s depolarization was clearly enhanced by 1 or 5 μm DHPG (Fig. 6A,C). Similarly, DSI after 0.1 s depolarization was enhanced by 5 μm DHPG (Fig. 6A,C). Application of 1 or 5 μm DHPG, however, had little effect on DSE (Fig. 6C). When the concentration was raised to 25 μm, DHPG effectively enhanced both DSI (n = 7) and DSE (n = 6) (Fig. 6B). As summarized in Figure 6C, both DSI and DSE underwent dose-dependent enhancement by DHPG with the sensitivity of DSI being higher than that of DSE. It should be noted that no apparent enhancement was seen in DSI after 5 s depolarization, because DSI magnitude is saturated by 5 s depolarization (Narushima et al., 2007). These results indicate that DSE exhibits lower sensitivity to DHPG than DSI for enhancement.
Figure 6.
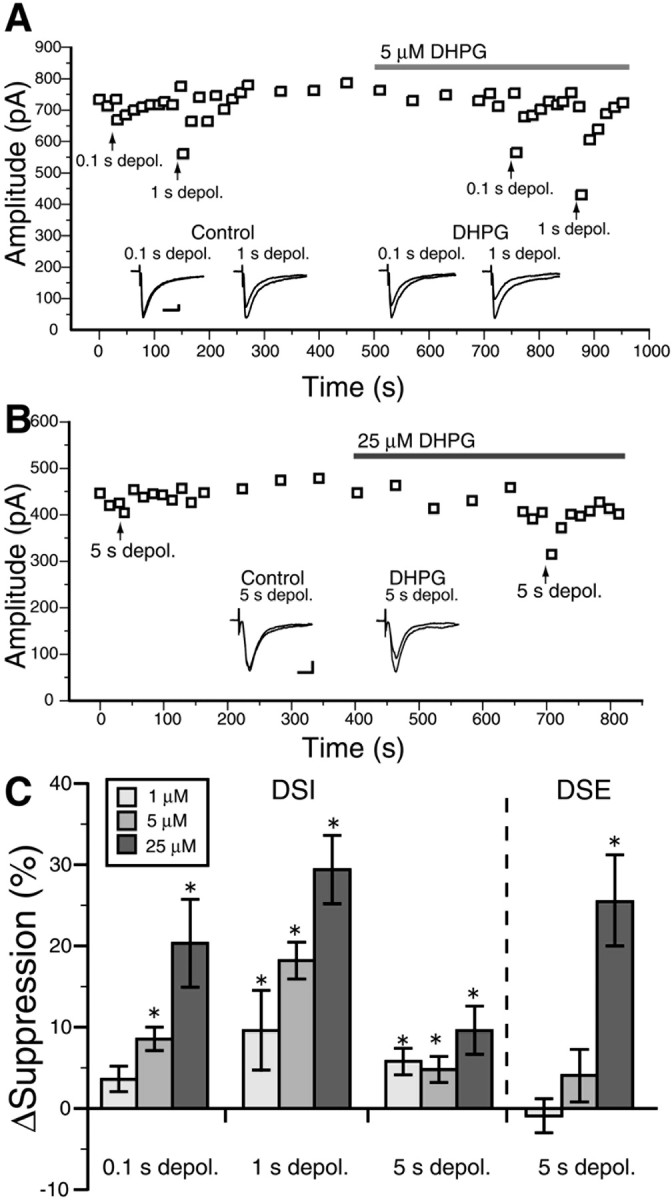
Activation of group I mGluRs causes dose-dependent enhancement of striatal DSI and DSE. A, Representative data demonstrating enhancement of DSI by 5 μm DHPG. Each point represents the average of three consecutive traces. Depolarizing pulses (depol.) with duration of 0.1 or 1 s were applied before and during DHPG application at the time points indicated with upward arrows. Inset, Sample IPSC traces showing DSI in the control external solution and in the presence of 5 μm DHPG. Calibration: 100 pA, 10 ms. B, Representative data demonstrating enhancement of DSE by 25 μm DHPG. Depolarizing pulses with 5 s duration were applied before and during DHPG application. Inset, Sample EPSC traces showing DSE before and after DHPG application. Calibration: 100 pA, 10 ms. C, Summary bar graphs showing dose-dependent enhancement of DSI and DSE by DHPG. The degree of enhancement was calculated as the change in the magnitude of DSI or DSE during DHPG application relative to the value before application (ΔSuppression).
DSE undergoes synergistic enhancement by coapplication of oxo-M and DHPG
Considering the wide distribution of M1 and mGluR5 on the extrasynaptic spine surface (Fig. 4), these two receptors could potentially exert synergistic effects on DSE enhancement. To test this possibility, we examined cooperative effect of simultaneous activation of M1 and mGluR5 on DSE. Application of subthreshold levels of DHPG alone (5 μm) or oxo-M alone (1 μm) could not enhance DSE after 5 s depolarization (summarized in Fig. 7B); the change in DSE magnitudes (ΔDSE) after application of agonists was 4.0 ± 3.3% (n = 6) and 2.7 ± 2.1% (n = 8), respectively, showing no significant enhancement (p = 0.35 for DHPG, p = 0.21 for oxo-M; compared with DSE magnitude before application). In contrast, simultaneous application of 5 μm DHPG and 0.5 μm oxo-M clearly enhanced DSE as exemplified in Figure 7A and summarized in Figure 7B (ΔDSE, 16.7 ± 2.0%, n = 7 vs DHPG alone, p = 0.02; vs oxo-M alone, p = 0.0026). The cooperative enhancement of DSE was effectively blocked by an M1-preferring mAChR antagonist, pirenzepine (0.7 ± 2.3%; n = 4; p = 0.0082) (Fig. 7B). These findings indicate that group I mGluRs and M1 act synergistically to facilitate endocannabinoid production in MS neurons.
Figure 7.
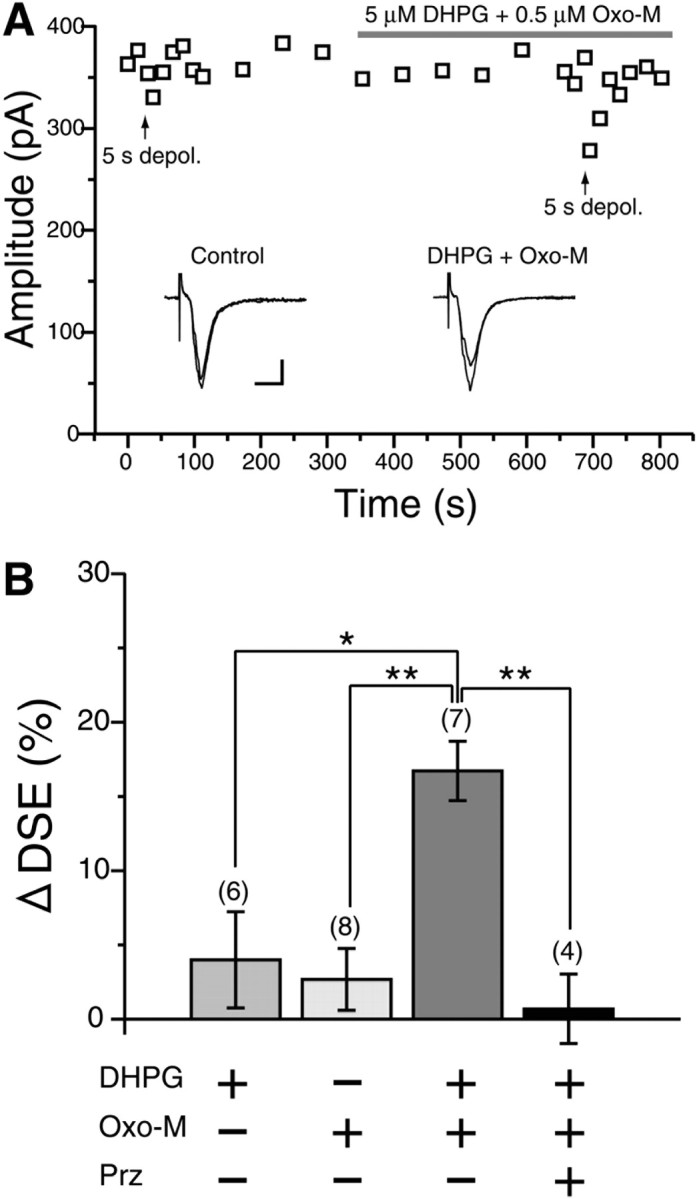
Cooperative enhancement of DSE by combined activation of M1 and group I mGluRs. A, Representative data showing enhancement of DSE by combined application of 5 μm DHPG and 0.5 μm oxo-M. Data are shown in a manner similar to Figure 6B. Calibration: 100 pA, 10 ms. depol., Depolarizing pulse. B, Summary bar graph showing enhancement of DSE by 5 μm DHPG alone, 1 μm oxo-M alone, coapplication of 5 μm DHPG and 0.5 μm oxo-M, and coapplication of DHPG and oxo-M in the presence of 1 μm pirenzepine (Prz).
CB1 cannabinoid receptor is highly expressed on two types of GABAergic axon terminals in the striatum
Our anatomical and physiological findings consistently indicate that 2-AG is the main endocannabinoid at both GABAergic and glutamatergic MS neuron synapses, except for the following point. Considering that spines are the postsynaptic targets of excitatory afferents (Fujiyama et al., 2004; Tepper et al., 2004), the lower sensitivity of DSE to DHPG than DSI for enhancement (Fig. 6) apparently contradicts the subcellular gradient of mGluR5 expression, being higher in spines than in dendritic shafts and somata (Fig. 3). A possible solution to resolve this apparent discrepancy would be that the density of CB1 might be different between excitatory and inhibitory afferents. We thus examined precise anatomical distribution of CB1 in neural elements of the striatum. For this analysis, we used an antibody against the C-terminal segment of CB1, whose specificity and sensitivity have been proven high enough to detect even a small amount of CB1 proteins expressed in hippocampal excitatory afferents of wild-type but not of CB1 knock-out mice (Fukudome et al., 2004; Katona et al., 2006; Kawamura et al., 2006).
By low-power observation over the forebrain, CB1 was detected at moderate levels in the striatum (supplemental Fig. S1H,I, available at www.jneurosci.org as supplemental material). Its cellular and synaptic expression was determined by immunofluorescence (Fig. 8) and immunoelectron (Fig. 9) microscopies. At high magnification, intense CB1 labeling was detected in large varicose fibers running throughout the neuropil (Fig. 8A′, arrows). Among these varicose fibers, thin structures with very faint CB1 labeling were also seen (Fig. 8A′, arrowheads). In addition, faint labeling was observed in perikarya of various striatal neurons. To unravel the identity of these CB1-immunoreactive profiles, we performed a series of double-immunofluorescence stainings in which neurochemical properties of these CB1-carrying elements were characterized using transmitter-specific axon terminal markers and cell-type-specific perikaryal markers. For this analysis, semithin cryosections (200 nm in thickness) were prepared to increase the spatial resolution (Fig. 8B–H), and the specificity of CB1 labeling was confirmed by using CB1 knock-out semithin sections as negative controls (supplemental Fig. S4A–G, available at www.jneurosci.org as supplemental material).
Figure 8.

CB1 is highly expressed in axons and terminals of medium spiny neurons and parvalbumin interneurons. Throughout this figure, CB1 is pseudocolored in red. A, Single immunofluorescence for CB1. Inset, Arrows and arrowheads indicate intense (putative inhibitory afferents) and weak (putative excitatory afferents) CB1 immunolabeling, respectively. B1–H2, Semithin cryosections subjected to double immunofluorescence for CB1 and terminal markers (green; B1,B2, vesicular GABA transporter VGAT; C1,C2, vesicular acetylcholine transporter VAChT; D1,D2, type 1 vesicular glutamate transporter VGluT1; E1,E2, type 2 vesicular glutamate transporter VGluT2; F1,F2, plasmalemmal dopamine transporter DAT; G1,G2, plasmalemmal serotonin transporter HTT; H1,H2, plasmalemmal norepinephrine transporter NET). Arrowheads and arrows indicate terminals judged to be positive and negative for CB1, respectively. I1–J2, Double immunofluorescence for CB1 and SP (green; I1, I2) or enkephalin (Enk) (green; J1, J2). Note low CB1 contents in neuropeptide-carrying perikarya (arrows) and high CB1 contents in neuropeptide-carrying axons (arrowheads). K1, K2, Triple immunofluorescence for CB1, PV (green), and VGAT (blue). Note low CB1 contents in PV-carrying perikarya (arrows) and high CB1 contents in PV/VGAT-carrying terminals (arrowheads), the latter surrounding a putative MS neuron (asterisk) in a basket-like manner. Scale bars: A, I2, J2, K2, 10 μm; A′, B2, C2, D2, E2, F2, G2, H2, 2 μm.
First, we tested whether the swelling portions of varicose fibers having high levels of CB1 (Fig. 8A′, arrows) belonged to GABAergic terminals. Many puncta labeled for inhibitory terminal marker VGAT were either positive for CB1 or connected to CB1-carrying fibers (Fig. 8B1,B2, arrowheads), whereas the rest of a few puncta were not associated with any CB1 immunolabeling (Fig. 8B1,B2, arrow). This finding was further supported at the cellular level, as perikaryal CB1 labeling was detected, although at low levels, in most of GAD-positive GABAergic perikarya inside the striatum (supplemental Fig. S4H, available at www.jneurosci.org as supplemental material). Next, we determined which types of GABAergic neurons expressed CB1. Of striatal GABAergic neurons, two types of MS neurons are also distinguished by selective expressions of SP (D1R neurons) and Enk (D2R neurons), whereas three types of GABAergic interneurons are distinguished by selective expressions of PV, NOS/somatostatin (Som), and calretinin (CR). CB1 was detected in SP-, Enk-, or PV-positive perikarya (Fig. 8I1–K2, arrows) and axons (Fig. 8I1–K2, arrowheads). Occasionally, CB1 was discernible in NOS-positive interneurons, but the levels were extremely low, if any (supplemental Fig. S4J,K, available at www.jneurosci.org as supplemental material). No CB1 immunostaining was seen in CR-containing interneurons (supplemental Fig. S4L, available at www.jneurosci.org as supplemental material). Therefore, among striatal GABAergic neurons, CB1 is mainly expressed by MS neurons and PV-positive interneurons.
To confirm the presence of CB1 on these GABAergic fibers, we prepared serial ultrathin sections subjected to double-labeling immunoelectron microscopy (Fig. 9A1–D2). The silver-enhanced immunogold method revealed that metal particles representing the precise subcellular localization of CB1 were observed on terminals labeled by immunoperoxidase for either SP (Fig. 9A1,A2), Enk (Fig. 9B1,B2), or PV (Fig. 9C1–D2). Furthermore, these labeled terminals formed symmetrical contact (Fig. 9A1–E, arrowheads) on spines, spiny dendrites, and somata with thin perikaryal rims and round nuclei, all suggesting that their postsynaptic targets are MS neurons.
CB1 is expressed at low levels on glutamatergic axon terminals in the striatum
The very faint CB1 immunolabeling in thin structures (Fig. 8A′, arrowheads) was also judged to be specific, because it was entirely missing in CB1 knock-out sections (supplemental Fig. S4A–G, available at www.jneurosci.org as supplemental material). To identify the structure, we performed double immunofluorescence staining using the following terminal markers: VGluT1 (glutamatergic terminal marker), VGluT2 (glutamatergic), VAChT (cholinergic), DAT (dopaminergic), HTT (serotonergic), and NET (norepinephrinergic) (Fig. 8C1–H2). Among these terminal markers, faint CB1 labeling was often detected on VGluT1-positive terminals only (Fig. 1D1,D2). Considering that VGluT1 is selectively present in glutamatergic afferents from the sensorimotor cortex, whereas VGluT2-containing glutamatergic axons are derived from the parafascicular thalamic nucleus (Fujiyama et al., 2004), the above results suggest that CB1 is expressed at low levels in corticostriatal glutamatergic afferents but not in other subcortical afferents. In support of this notion, moderate levels of CB1 mRNA were detected in layer V neurons of the sensorimotor cortex, which send corticostriatal afferents (supplemental Fig. S5C,D, available at www.jneurosci.org as supplemental material). In contrast, CB1 mRNA was at the background level, if any, in neurons of the parafascicular thalamic nucleus, substantia nigra pars compacta, dorsal raphe, and locus ceruleus, which supply glutamatergic, dopaminergic, serotonergic, and norepinephrinergic afferents, respectively, to the striatum (supplemental Fig. S5E–O, available at www.jneurosci.org as supplemental material).
CB1 expression in corticostriatal afferents was verified by immunogold labeling for CB1. Metal particles representing the localization of CB1 were indeed found on nerve terminals forming asymmetrical synapses onto dendritic spines (Fig. 9E, arrow). Quantification of metal particles on electron micrographs revealed that the density of CB1-labeling was 3.6-fold higher on axon terminals forming symmetrical synapses than on boutons making asymmetrical synapses (Fig. 9F) (p < 0.01, Student's t test). The labeling at asymmetrical synapses was judged to be specific, because the density was significantly higher than the control level as determined from the density at asymmetrical synapses of CB1 knock-out mice (Fig. 9F) (p < 0.01).
Together, CB1 cannabinoid receptor in the striatum is localized at high levels on GABAergic afferents forming PV interneuron–MS neuron (PV–MS) synapses and MS neuron collateral–MS neuron (MS–MS) synapses, as well as at low levels on glutamatergic afferents forming corticostriatal synapses.
Discussion
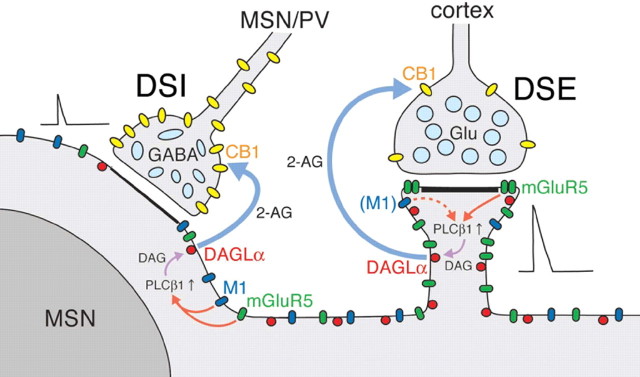
Through molecular, anatomical, and physiological approaches, our findings highlight that the 2-AG-mediated retrograde signaling system is organized in the striatum in a highly regulated manner, as schematically illustrated in Figure 10.
Figure 10.
A schematic illustration showing the molecular, anatomical, and physiological organization of 2-AG-mediated retrograde signaling at excitatory (right) and inhibitory (left) synapses onto medium spiny neuron (MSN). At corticostriatal synapses, coincidental depolarization and mGluR5 activation are essential for PLCβ1/DAGLα-mediated production of 2-AG to induce DSE because of low CB1 levels in excitatory corticostriatal afferents. Induction of mGluR5-assisted DSE is further facilitated with the aid of M1, although M1 activation alone cannot enhance DSE. This DSE facilitation mechanism will lead to the suppression of the hyperactivity of the MS neuron. At MSN–MSN and PV interneuron–MSN synapses, DSI can be induced by depolarization alone, attributable to high CB1 levels in these inhibitory afferents. Nevertheless, coactivation of mGluR5 or M1 robustly enhances the magnitude of DSI, which should lead to the increase of the excitability and striatal output of the MS neuron.
Comparison with previous studies on striatal CB1 expression
High CB1 expression on inhibitory axons/terminals of PV interneurons and MS neurons is compatible with previous studies using double-labeling in situ hybridization (Marsicano and Lutz, 1999; Hohmann and Herkenham, 2000) and with an immunogold study showing dense distribution of CB1 on GABAergic axons and terminals in the striatum (Matyas et al., 2006). Low CB1 immunoreactivity in NOS-positive interneurons (supplemental Fig. S4J,K, available at www.jneurosci.org as supplemental material) is not incompatible with negativity of CB1 mRNA in Som-positive interneurons (Hohmann and Herkenham, 2000); rather, the two studies are consistent in that CB1 expression in this interneuron is around the threshold level. Here we have further demonstrated that the CB1-rich GABAergic elements are the afferents of PV–MS and MS–MS synapses and that CB1 is also expressed on corticostriatal excitatory synapses. Importantly, these anatomical results are fully compatible with the electrophysiological findings that ERS is observed at PV–MS (Freiman et al., 2006; Narushima et al., 2006b), MS–MS (Freiman et al., 2006), and corticostriatal synapses (Gerdeman and Lovinger, 2001; Huang et al., 2001; Kreitzer and Malenka, 2005; Narushima et al., 2006a).
In contrast, our predominant CB1 staining in presynaptic elements is quite different from intense perikaryal staining of various striatal neurons, as shown by Fusco et al. (2004). Intense perikaryal labeling was reported for CCK-positive interneurons in the hippocampus, cerebral cortex, and amygdala (Katona et al., 1999, 2001; Hajos et al., 2000; Fukudome et al., 2004; Bodor et al., 2005) but has never been seen in the striatum (Julian et al., 2003; Matyas et al., 2006) (Fig. 8). The lack of CB1 in the postsynaptic density and dopaminergic afferents in our study is also incompatible with the study by Kofalvi et al. (2005). Although reasons for these discrepancies remain unclear, we verified the specificity of our CB1 immunohistochemistry by negative immunostaining in the CB1-knock-out striatum (Fig. 9F, supplemental Figs. S1H, S4A–G, available at www.jneurosci.org as supplemental material).
2-AG-mediated retrograde suppression at corticostriatal synapse
In addition to CB1 expression on corticostriatal afferents, we have revealed for the first time that DAGLα is distributed at the highest level on spines of MS neurons. As for mGluR5, our observations of somatodendritic distribution in the order of spine > dendritic shaft > soma, predominant extrasynaptic expression, and perisynaptic accumulation at asymmetrical synapses are consistent with previous studies (Paquet and Smith, 2003; Mitrano and Smith, 2007). This molecular organization will explain the electrophysiological findings that corticostriatal excitatory transmission is suppressed by CB1 agonists (Gerdeman and Lovinger, 2001; Huang et al., 2001) and that DSE and endocannabinoid-dependent LTD are induced in MS neurons only when postsynaptic depolarization or L-type calcium channel activation is combined with group I mGluR activation (Kreitzer and Malenka, 2005; Narushima et al., 2006a) (Fig. 6). Such cooperativity is also found for hippocampal neurons (Varma et al., 2001; Ohno-Shosaku et al., 2002) and cerebellar Purkinje cells (Brenowitz and Regehr, 2005; Maejima et al., 2005). PLCβ is sensitive to both intracellular calcium levels and Gq activity and functions as a coincidence detector of the two events (Hashimotodani et al., 2005; Maejima et al., 2005).
In awake animals, MS neurons are almost quiescent (i.e., down-state) when the animals are at rest. MS neurons display transient high-frequency firing (up-state) in response to synchronous excitatory inputs from the sensorimotor cortex, when the animals initiate movements (Hikosaka and Wurtz, 1989). As cortical activity is increased, high-frequency activation of ionotropic and metabotropic glutamate receptors activates PLCβ supralinearly (most likely PLCβ1 in MS neurons) (Watanabe et al., 1998), elevates DAG levels in the activated spines, and produces 2-AG from DAG by DAGLα. Then, 2-AG travels backwards to suppress glutamate release from the terminals (Fig. 10, right). Intriguingly, in slice preparation, DSE cannot be induced by depolarization alone and requires coactivation of group I mGluRs (Narushima et al., 2006a). Because CB1 density is low on corticostriatal afferents, relatively high concentration of 2-AG around corticostriatal excitatory synapses must be required for DSE induction. The abundance of mGluR5 and DAGLα in spines should help 2-AG synthesis beyond the threshold, when postsynaptic depolarization and mGluR5 activation coincide. Hence, such molecular arrangements around corticostriatal synapses should enable this local feedback loop to operate only after robust cortical excitation, which leads to suppression of hyperactivity of the MS neuron.
2-AG-mediated retrograde suppression at PV–MS and MS–MS synapses
Inhibitory synaptic transmissions at PV–MS and MS–MS synapses are also sensitive to CB1 agonists (Szabo et al., 1998; Freiman et al., 2006; Narushima et al., 2006b), and the magnitude of DSI is enhanced by activation of group I mGluRs (Freiman et al., 2006) (Fig. 5C,D). In contrast to DSE, DSI in MS neurons can be induced by depolarization alone (Narushima et al., 2006b). This may be relevant to abundant CB1 at these inhibitory synapses. Moreover, robust DSI enhancement by activation of group I mGluRs or M1 (Narushima et al., 2007) (Fig. 5C–F) and the distribution of DAGLα, mGluR5, and M1 around inhibitory synapses (Narushima et al., 2007) (Figs. 2, 3) point to the physiological importance of coincidental activation of these Gq-coupled receptors for DSI induction (Fig. 10, left), as is the case for DSE induction. Indeed, ambient acetylcholine derived from cholinergic interneurons is involved in tonic enhancement of DSI in MS neurons (Narushima et al., 2007).
PV or fast-spiking interneurons receive cortical excitatory inputs and exert powerful inhibition onto the MS neuron through their pericellular basket formation and through their electrical coupling via gap junction (Kita et al., 1990; Koos and Tepper, 1999; Bolam et al., 2000). Even a single spike of PV interneuron can delay or prevent spiking in an MS neuron (Koos and Tepper, 1999). Therefore, PV–MS synapse constitutes a powerful feedforward inhibitory circuits, and DSI at this synapse leads to the increase of the excitability of the MS neuron. On the other hand, MS neuron collaterals form numerous inhibitory synapses onto dendritic shafts and spines of MS neurons (Kubota and Kawaguchi, 2000; Wilson, 2004). Compared with PV–MS synapses, MS–MS synapses produce smaller IPSCs when measured at the soma and are thought to modulate dendritic integration and synaptic plasticity of corticostriatal and thalamostriatal synapses and to attenuate backpropagation of action potentials into dendrites (Tepper et al., 2004). Therefore, DSI of MS–MS synapse may augment excitability of the MS neuron less effectively than that of PV–MS synapse but would elaborate on information processing in local dendrites of MS neurons.
Differential but synergistic modulation by glutamatergic and cholinergic inputs on DSE enhancement
Although group I mGluR agonist DHPG enhances both DSI and DSE, mAChR agonist oxo-M enhances DSI only (Narushima et al., 2006a) (Fig. 5C–F). However, oxo-M can readily enhance DSE when coadministered with DHPG (Fig. 7). This finding suggests that induction threshold for striatal ERS is modulated differentially but synergistically depending on cholinergic and cortical activities. Tonically active cholinergic interneurons in primates change their firings in response to reward-related or aversive stimuli (Apicella, 2002). Cholinergic interneuron activity is also influenced by nigrostriatal dopaminergic and thalamostriatal glutamatergic inputs (Aosaki et al., 1994; Bennett and Wilson, 1998; Watanabe and Kimura, 1998). Therefore, it can be assumed that as cholinergic activity is elevated alone, the threshold for DSI decreases preferentially, whereas that for both DSI and DSE decreases when cholinergic and corticostriatal activities are elevated simultaneously. Thus, the balance between cholinergic and cortical inputs will determine the degree of ERS of inhibition and excitation and should modulate striatal output.
In hippocampal pyramidal cells and Purkinje cells, mGluR1α and mGluR5 are accumulated at the perisynaptic annulus of excitatory synapses (Baude et al., 1993; Nusser et al., 1994; Lujan et al., 1996, 1997). These group I mGluRs form complexes coimmunoprecipitated with inositol 1,4,5-trisphosphate receptor (IP3R) via scaffolding protein Homer (Brakeman et al., 1997; Tu et al., 1998). Homer further binds to ionotropic glutamate receptors via Shank and GRIP (glutamate receptor-interacting protein) (Dong et al., 1997; Sheng and Kim, 2000; Uemura et al., 2004). Furthermore, PLCβ is accumulated at the perisynaptic region and forms complexes coimmunoprecipitated with mGluR1α, IP3R, and Shank (Nakamura et al., 2004; Hwang et al., 2005; Nomura et al., 2007). At excitatory synapses, group I mGluRs and their downstream molecules are thus physically interacted and recruited to the perisynaptic region, presumably to increase the efficiency and specificity of signal transduction. The same mechanism may recruit mGluR5 to the perisynaptic region of corticostriatal synapses (Paquet and Smith, 2003) (Fig. 4A) but might exclude M1 from there (Fig. 4B). In contrast, mGluR5 is distributed more loosely around symmetrical synapses, regardless of synaptic and extrasynaptic membranes (Paquet and Smith, 2003) (our unpublished data). We assume that different somatodendritic and perisynaptic distributions between mGluR5 and M1 (Fig. 10) may cause different extents of receptor–effector coupling and underlie differential effects of DHPG and oxo-M on DSE enhancement.
In conclusion, our findings provide evidence that a set of receptors and enzymes involved in 2-AG-mediated retrograde signaling are arranged in the striatum to be able to modulate effectively the excitability of the MS neuron depending on cortical activity and cholinergic tone.
Footnotes
This work was supported in part by grants-in-aid for Scientific Research on Priority Areas (17023001 to M.W.; 17023021 to M.K.), for Scientific Research (B) (17300108 to M.W.), and for Scientific Research (S) (17100004 to M.K.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; by Hungarian Scientific Research Fund Grant F046407; and by Egészségügyi Tudományos Tanács Grant 561/2006 (I.K.). M.N. was a recipient of the Research Fellowship for Young Scientists from the Japan Society for the Promotion of Science (16·54131). I.K. is a grantee of the János Bolyai scholarship.
References
- Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265:412–415. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- Apicella P. Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. Eur J Neurosci. 2002;16:2017–2026. doi: 10.1046/j.1460-9568.2002.02262.x. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Synaptic regulation of action potential timing in neostriatal cholinergic interneurons. J Neurosci. 1998;18:8539–8549. doi: 10.1523/JNEUROSCI.18-20-08539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PAC, Brevan MD. Synaptic organization of the basal ganglia. J Anat. 2000;1196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci. 2003;23:6373–6384. doi: 10.1523/JNEUROSCI.23-15-06373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–431. doi: 10.1016/j.neuron.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Menetrey A, Charpier S. The lamellar organization of the rat substantia nigra pars reticulata: segregated patterns of striatal afferents and relationship to the topography of corticostriatal projections. Neuroscience. 1996;73:761–781. doi: 10.1016/0306-4522(96)00088-7. [DOI] [PubMed] [Google Scholar]
- Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Freiman I, Anton A, Monyer H, Urbanski MJ, Szabo B. Analysis of the effects of cannabinoids on identified synaptic connections in the caudate-putamen by paired recordings in transgenic mice. J Physiol (Lond) 2006;575:789–806. doi: 10.1113/jphysiol.2006.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Kuramoto E, Okamoto K, Hioki H, Furuta T, Zhou L, Nomura S, Kaneko T. Presynaptic localization of an AMPA-type glutamate receptor in corticostriatal and thalamostriatal axon terminals. Eur J Neurosci. 2004;20:3322–3330. doi: 10.1111/j.1460-9568.2004.03807.x. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Fusco FR, Martorana A, Giampa C, De March Z, Farini D, D'Angelo V, Sancesario G, Bernardi G. Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse. 2004;53:159–167. doi: 10.1002/syn.20047. [DOI] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;27:1211–1219. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. The basal ganglia. Rev Oculomot Res. 1989;3:257–281. [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol (Lond) 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JI, Kim HS, Lee JR, Kim E, Ryu SH, Suh PG. The interaction of phospholipase C-β3 with Shank2 regulates mGluR-mediated calcium signal. J Biol Chem. 2005;280:12467–12473. doi: 10.1074/jbc.M410740200. [DOI] [PubMed] [Google Scholar]
- Julian MD, Martin AB, Cuellar B, Rodriguez De Fonseca F, Navarro M, Moratalla R, Garcia-Segura LM. Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience. 2003;119:309–318. doi: 10.1016/s0306-4522(03)00070-8. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. CB1 is the major cannabinoid receptor at excitatory presynaptic site in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kosaka T, Heizmann CW. Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res. 1990;536:1–15. doi: 10.1016/0006-8993(90)90002-s. [DOI] [PubMed] [Google Scholar]
- Kofalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlagh B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci. 2000;20:375–386. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1α, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cβ4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Di Marzo V, Gessa GL, Pistis M. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Miura E, Fukaya M, Sato T, Sugihara K, Asano M, Yoshioka K, Watanabe M. Expression and distribution of JNK/SAPK-associated scaffold protein JSAP1 in developing and adult mouse brain. J Neurochem. 2006;97:1431–1446. doi: 10.1111/j.1471-4159.2006.03835.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Fukaya M, Shimizu H, Watanabe M. Subtype switching of vesicular glutamate transporters at parallel fibre-Purkinje cell synapses in developing mouse cerebellum. Eur J Neurosci. 2003;17:2563–2572. doi: 10.1046/j.1460-9568.2003.02698.x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, Watanabe M. Signaling complex formation of phospholipase Cβ4 with metabotropic glutamate receptor type 1α and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci. 2004;20:2929–2944. doi: 10.1111/j.1460-9568.2004.03768.x. [DOI] [PubMed] [Google Scholar]
- Narushima M, Hashimoto K, Kano M. Endocannabinoid-mediated short-term suppression of excitatory synaptic transmission to medium spiny neurons in the striatum. Neurosci Res. 2006a;54:159–164. doi: 10.1016/j.neures.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Hashimoto K, Watanabe M, Kano M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur J Neurosci. 2006b;24:2246–2252. doi: 10.1111/j.1460-9568.2006.05119.x. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Fukaya M, Matsui M, Manabe T, Hashimoto K, Watanabe M, Kano M. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S, Fukaya M, Tsujioka T, Wu D, Watanabe M. Phospholipase Cβ3 is distributed in both somatodendritic and axonal compartments and localized around perisynapse and smooth endoplasmic reticulum in mouse Purkinje cell subsets. Eur J Neurosci. 2007;25:659–672. doi: 10.1111/j.1460-9568.2007.05334.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mulvihill E, Streit P, Somogyi P. Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience. 1994;61:421–427. doi: 10.1016/0306-4522(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci. 2002;15:953–961. doi: 10.1046/j.1460-9568.2002.01929.x. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Hashimotodani Y, Maejima T, Kano M. Calcium signaling and synaptic modulation: regulation of endocannabinoid-mediated synaptic modulation by calcium. Cell Calcium. 2005;38:369–374. doi: 10.1016/j.ceca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Palomaki VA, Lehtonen M, Savinainen JR, Laitinen JT. Visualization of 2-arachidonoylglycerol and cannabinoid CB(1) receptor activity in rat brain cryosections by functional autoradiography. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2006.04403.x. in press. [DOI] [PubMed] [Google Scholar]
- Paquet M, Smith Y. Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J Neurosci. 2003;23:7659–7669. doi: 10.1523/JNEUROSCI.23-20-07659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Hanley JJ, Deniau JM, Bolam JP. Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J Neurosci. 2002;22:8158–8169. doi: 10.1523/JNEUROSCI.22-18-08158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Lovinger DM. Induction of striatal long-term synaptic depression by moderate frequency activation of cortical afferents in rat. J Physiol (Lond) 2005;562:245–256. doi: 10.1113/jphysiol.2004.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim E. The Shank family of scaffold proteins. J Cell Sci. 2000;113:1851–1856. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, Fukaya M, Shigemoto R, Watanabe M, Somogyi P. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19:552–569. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Szabo B, Dorner L, Pfreundtner C, Norenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- Szabo B, Urbanski MJ, Bisogno T, Di Marzo V, Mendiguren A, Baer WU, Freiman I. Depolarization-induced retrograde synaptic inhibition in the mouse cerebellar cortex is mediated by 2-arachidonoylglycerol. J Physiol. 2006;577:263–280. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Nakagawa S, Kushiya E, Yamasaki M, Fukaya M, Iwanaga T, Simon MI, Sakimura K, Kano M, Watanabe M. Gq protein alpha subunits Gαq and Gα11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur J Neurosci. 2000;12:781–792. doi: 10.1046/j.1460-9568.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Uemura T, Mori H, Mishina M. Direct interaction of GluRδ2 with Shank scaffold proteins in cerebellar Purkinje cells. Mol Cell Neurosci. 2004;26:330–341. doi: 10.1016/j.mcn.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21(RC188):1–5. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kimura M. Dopamine receptor-mediated mechanisms involved in the expression of learned activity of primate striatal neurons. J Neurophysiol. 1998;79:2568–2580. doi: 10.1152/jn.1998.79.5.2568. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Nakamura M, Sato K, Kano M, Simon MI, Inoue Y. Patterns of expression for the mRNA corresponding to the four isoforms of phospholipase Cβ in mouse brain. Eur J Neurosci. 1998;10:2016–2025. doi: 10.1046/j.1460-9568.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Basal ganglia. In: Shephard GM, editor. The synaptic organization of the brain. Ed 5. Oxford: Oxford UP; 2004. pp. 361–414. [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yamada K, Fukaya M, Shimizu H, Sakimura K, Watanabe M. NMDA receptor subunits GluRε1, GluRε3 and GluRζ1 are enriched at the mossy fibre-granule cell synapse in the adult mouse cerebellum. Eur J Neurosci. 2001;13:2025–2036. doi: 10.1046/j.0953-816x.2001.01580.x. [DOI] [PubMed] [Google Scholar]
- Yan Z, Flores-Hernandez J, Surmeier DJ. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience. 2001;103:1017–1024. doi: 10.1016/s0306-4522(01)00039-2. [DOI] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci USA. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-α around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]