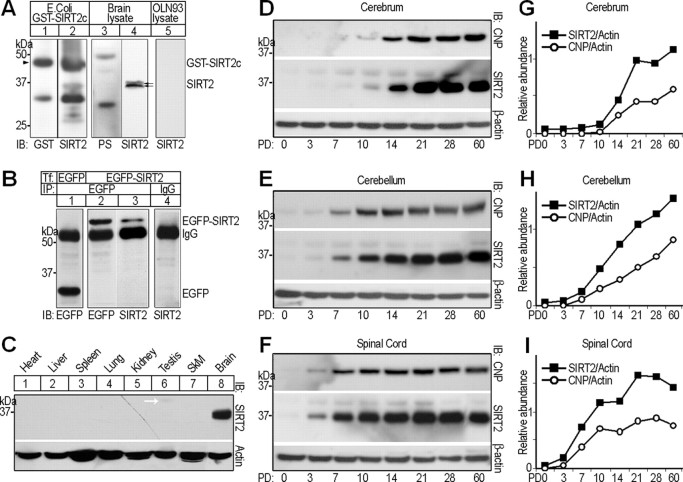
Figure 1.
Molecular features of rat SIRT2. A, Western blots, showing specificity tests of rabbit polyclonal anti-SIRT2 antibody. Labels above and below blots indicate the protein sample origins and antibodies used for immunoblotting detection (IB), respectively. GST-SIRT2c, Recombinant GST-SIRT2 C terminus (209–351) protein expressed in Escherichia coli. PS, Preimmunization serum. The double arrows indicate the SIRT2 doublet detected by rabbit anti-SIRT2. The arrowhead points to the position of GST-SIRT2c. B, OLN-93 cells were transiently transfected (Tf) with either pEGFP-C1 vector (lane 1) or pEGFP-Sirt2 (lanes 2–4). Forty-eight hours after transfection, lysates of the cells were subjected to immunoprecipitation (IP) using anti-EGFP or irrelevant IgG, followed by IB with antibodies indicated below. C, Multitissue Western blot probed with rabbit polyclonal anti-SIRT2 antibody. The bottom panel shows the pan-actin immunoreactivity as loading control. SkM, Skeleton muscle. D–I, Western blot analyses comparing the expression profiles of SIRT2 and CNP in postnatal day 0, 3, 7, 10, 14, 21, 28, or 60 rat cerebrum, cerebellum, and spinal cord, respectively. Each lane was loaded with 20 μg of soluble tissue lysate. Signals for β-actin served as loading controls. Quantitative analyses of expression levels of SIRT2 and CNP relative to β-actin in the different CNS regions are shown on the right (G–I). PD, Postnatal day.