Figure 7.
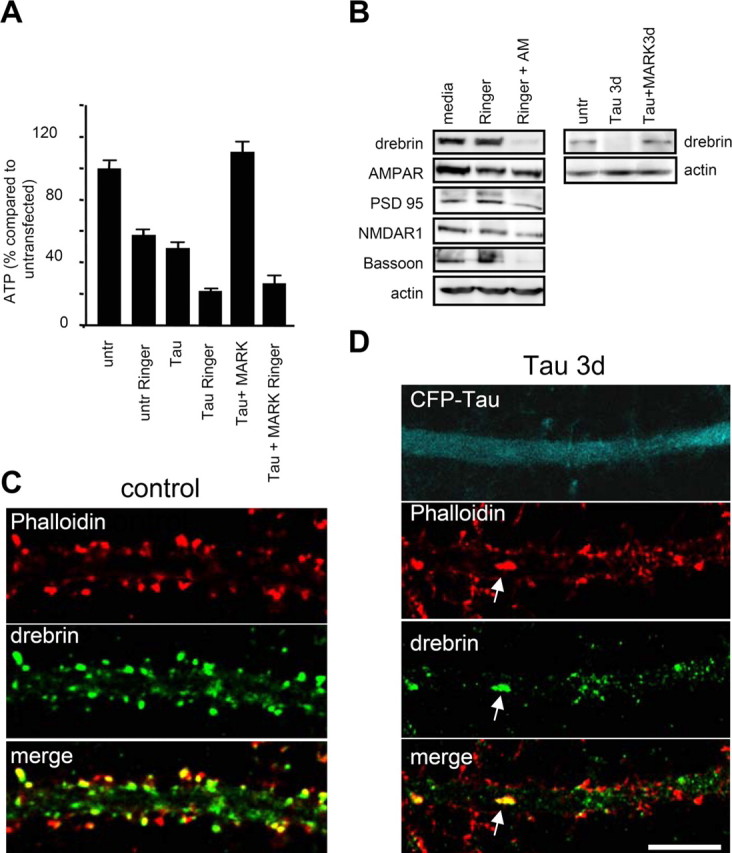
ATP decrease caused by tau transfection leads to a decrease in synaptic proteins. A, ATP levels in tau transfected and tau plus MARK2-transfected cultures. After 3 d of tau transfection, ATP decreases by one-half (50 ± 7%; n = 5; *p < 0.001), but remains high in tau plus MARK2 doubly transfected cultures (114 ± 7%; n = 3; *p < 0.05). To assess the amount of ATP synthesized by glycolysis, cultures were treated for 2.5 h with Ringer solution before ATP measurements. ATP levels of untreated cultures were reduced by one-half (57 ± 4%; n = 8; *p < 0.001), indicating that glycolysis and mitochondrial synthesis contribute about equally to the ATP production. Tau-transfected cultures in Ringer show an additional decrease by one-half. B, Energy deprivation with 1 μm antimycin (AM) and Ringer for 2.5 h cause a strong protein downregulation of drebrin, bassoon and a slight downregulation of NMDAR1, but not of PSD95 and AMPAR in Western blots. Tau expression also results in a reduction of drebrin after 3 d of transfection. Double transfection with tau and MARK2 prevents this loss. C, D, Immunostaining of drebrin in cultures transfected for 3 d (D) shows a strong reduction of drebrin staining compared with control (C). Drebrin staining disappears from the spines and often colocalizes with F-actin in the dendritic shaft (arrow). Scale bar, 10 μm. Error bars indicate SE.
