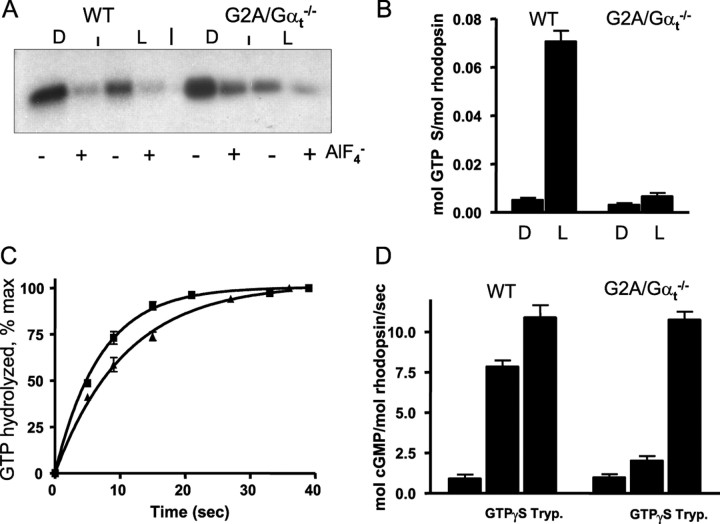
Figure 3.
A, Pertussis toxin-catalyzed ADP ribosylation of Gαt and GαtG2A. Dark-isolated (D) or bleached (L) WT ROS (0.6 μm R) and GαtG2A/Gαt−/− ROS (6 μm R) were incubated with preactivated pertussis toxin (3 μg/ml) and 5 μm [32P]NAD for 1 h at 25°C in the presence or absence of 30 μm AlCl3 and 10 mm NaF. The reactions were stopped by the addition of SDS-PAGE sample buffer and analyzed by SDS-PAGE and autoradiography. B, Light-dependent binding of GTPγS to control WT and GαtG2A/Gαt−/− ROS. Mouse ROS (10 μl, 10 μm R) were mixed with 5 μl of 10 μm [35S]GTPγS under infrared illumination. After 10 s incubation, the amount of bound GTPγS was determined in a 6 μl aliquot by the nitrocellulose filter binding assay. The rest of the sample was bleached to determine the GTPγS binding in the light. Error bars indicate SE (n = 3). C, Transducin GTPase activities in ROS from control WT and GαtG2A/Gαt−/− mice. The single-turnover GTPase measurements were initiated by mixing photobleached ROS (12 μl) containing 10 μm rhodopsin with 3 μl of 0.1 μm [γ-32P]GTP. The time course of 32Pi formation was determined using the activated charcoal procedure after the reactions were stopped with perchloric acid. The GTPase rate constants from single-phase exponential fits for the control (■) and GαtG2A/Gαt−/− (▴) ROS were 0.15 ± 0.0 and 0.09 ± 0.00 s−1 (mean ± SE; n = 3). D, PDE6 activation in ROS membranes. cGMP hydrolysis was measured in suspensions of bleached ROS membranes (3 μm R) in the absence or presence of 10 μm GTPγS using a 30 s incubation with 4 mm [3H]cGMP. Trypsin (Tryp.)-treated ROS were prepared as described in Materials and Methods. Error bars indicate SE (n = 3).