Figure 6.
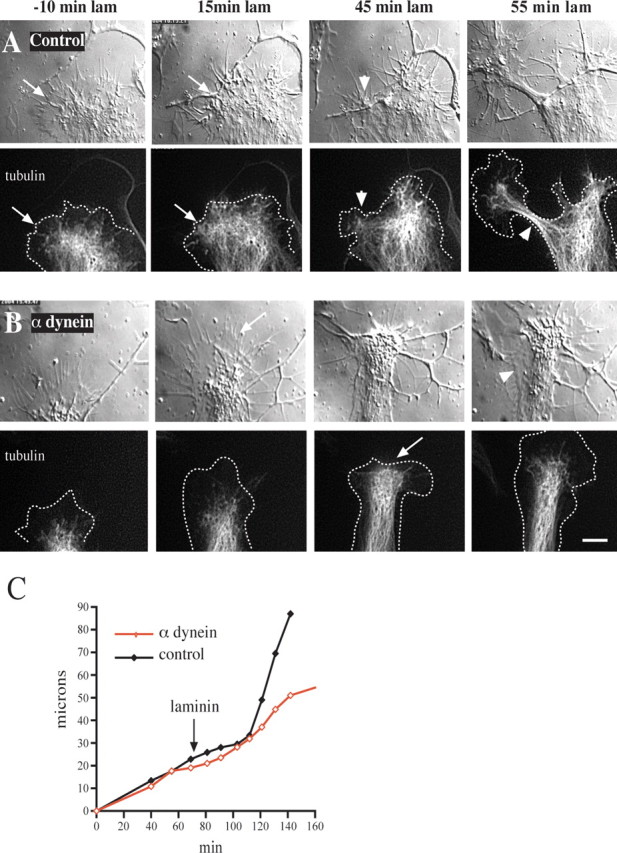
Effect of anti-dynein IC injection on growth cone morphology and microtubule distribution. Chick DRG neurons grown on a polyamine substrate were transfected with cDNA encoding EGFP-α-tubulin. Injected and uninjected control cells then were treated with laminin and monitored by DIC and fluorescence microscopy. A, Control uninjected cell. At 30 min after antibody injection and 10 min before laminin addition the microtubules are confined mainly to the central zone some distance from the leading edge (arrows in −10 min laminin). At 15 min after laminin treatment the microtubules have advanced to the leading edge (arrow). At 45 min after laminin treatment foci of microtubules have formed at the leading edge, around which the membrane also has begun to constrict (arrowhead). By 55 min a rapidly advancing neurite with a tightly constricted membrane and tightly bundled microtubules has emerged (arrowhead). B, Growth cone of a cell injected with anti-dynein IC antibody. At 30 min after antibody injection and 10 min before laminin treatment the growth cone displays normal spread morphology with microtubules confined to the central zone. At 15 min after laminin treatment the microtubules fail to reach the leading edge (arrow), and the growth cone advances at its initial slow rate. At 45 min after laminin treatment the microtubules near the leading edge but do not form foci (arrow). At 55 min after laminin treatment the peripheral zone has become reestablished. Lamellipodial protrusions also can be observed along that portion of the neurite that formed during the period of observation (arrowhead). Dotted lines in tubulin images represent the cell boundary. Scale bar, 10 μm. C, Growth rates of the growth cones depicted in A and B. The two growth cones initially advance at a comparable rate before laminin. After laminin treatment the control growth cone speeds up approximately threefold, whereas the rate of advance of the injected growth cone remains unchanged.
