Figure 2.
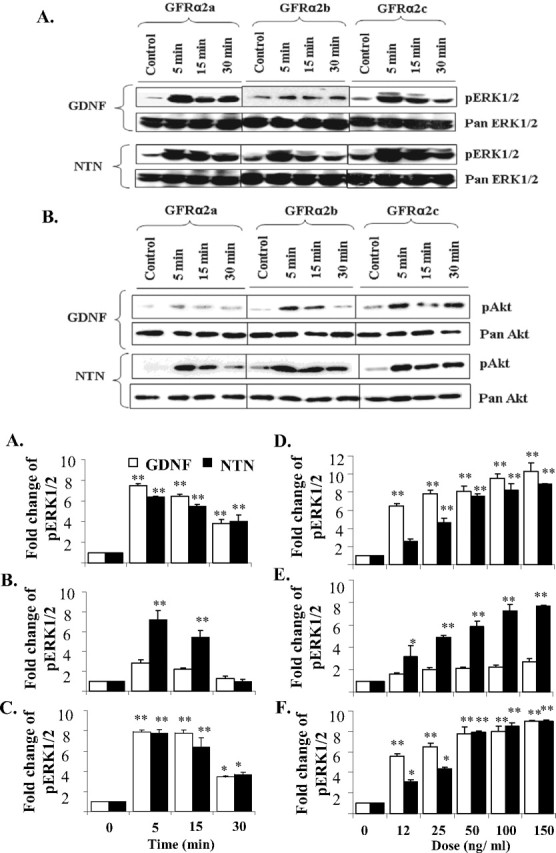
Activations of ERK1/2 and Akt in GFRα2 isoforms transfected Neuro2A cells when stimulated by either GDNF or NTN. Cells were stimulated in serum-free medium, with or without GDNF or NTN (50 ng/ml), for the period of time indicated. A, B, Five micrograms of protein were loaded and separated by SDS electrophoresis, and phosphorylated ERK1/2 (pERK1/2; A) or Akt (pAkt; B) was then detected by Western blot. Blots were stripped and reprobed with pan antibody as loading controls. C–E, Kinetics of GDNF and NTN induced ERK1/2 activations in GFRα2a (C), GFRα2b (D), and GFRα2c (E). Cells were treated with 50 ng/ml GDNF or NTN for 5, 15, and 30 min. F–H, Dose responses of the activation of ERK1/2 when stimulated with GDNF or NTN in GFRα2a (F), GFRα2b (G), and GFRα2c (H) isoforms. Cells were stimulated for 10 min with ligand at various doses. For kinetic and dose–response studies, 5 μg of protein was loaded per well for dot blot quantification of phospho-ERK1/2 (pERK1/2). The means ± SD were calculated from results obtained in triplicates. Significant differences in fold change of pERK1/2 between ligand stimulated and control were calculated using the paired Student's t test. A value of p < 0.05 was considered significant (**p < 0.001; *p < 0.05). Experiments were repeated three times with two independent clones with similar results.
