Figure 5.
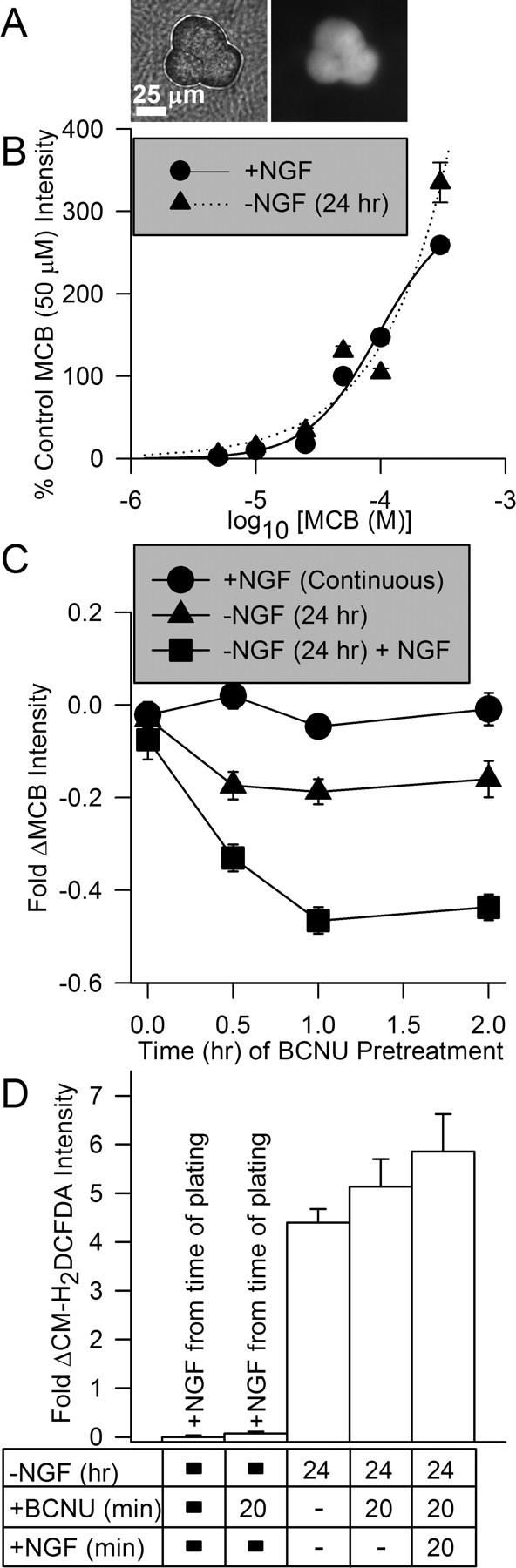
Activation of glutathione redox cycling was responsible for the rapid suppression of ROS by NGF. A, Photomicrographs showing phase-contrast (left) and fluorescent (right) images of a cluster of three sympathetic neuronal somas stained with MCB (300 μm). B, Staining of both NGF-replete and -deprived (24 h) cells with MCB was identical. These data indicate that the activity of glutathione S-transferase was not affected by NGF withdrawal. They also indicate that GSH concentration was similar in both conditions. Data was normalized to that of NGF-replete cells loaded with 50 μm MCB. Curves are best least-squares fits of two parameter logistic equations to the data. n = 35–284 neurons. C, Inhibition of glutathione reductase increased loss of GSH in NGF-deprived cells. Treatment of cultures with the glutathione reductase inhibitor BCNU (5 μm) did not affect MCB staining (300 μm) of neurons that had been maintained in media containing NGF. It did decrease MCB staining in NGF-deprived neurons, consistent with increased ROS in those cells and a more active glutathione pathway for detoxifying the ROS. Cultures deprived of NGF for 24 h and then exposed to NGF during the time of BCNU treatment exhibited greatly decreased MCB staining compared with control cells and cells deprived of NGF for 24 h. Data were normalized to MCB intensity of NGF-maintained cultures not treated with BCNU. n = 26–107 neurons. D, Suppression of glutathione cycling by BCNU (5 μm) prevented suppression of ROS by NGF readdition. Neurons deprived of NGF for 24 h were loaded with CM-H2DCFDA in the presence of NGF or NGF and BCNU. n = 87–283 neurons.
