Figure 5.
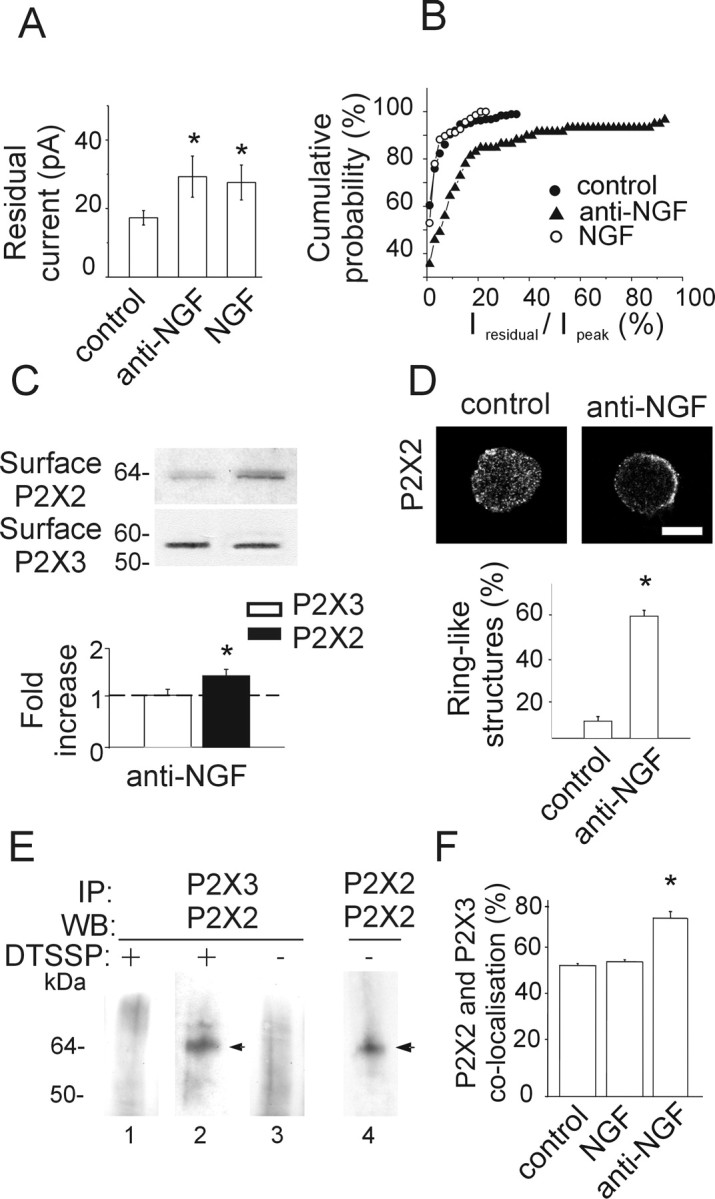
Anti-NGF treatment increases expression of heteromeric P2X2/3 receptors. A, Histograms showing residual current amplitude in control (n = 187) and after anti-NGF (n = 59) or NGF (n = 70) treatment. *p < 0.05. B, Cumulative probability plot of the ratio Iresidual/Ipeak for control condition (filled circles; n = 224), anti-NGF antibody treatment (filled triangles; n = 79), and NGF treatment (open circles; n = 85). Note that antibody-treated, but not NGF-treated, neurons show larger Iresidual, indicative of higher contribution by heteromeric P2X2/3 receptors. The activity of heteromeric P2X2/3 receptors is estimated as the ratio between the amplitude of the steady-state Iresidual at the end of α,β-meATP application and the one of the peak current (Ipeak). C, Example of biotinylation experiments showing that NGF deprivation treatment increases the surface expression of the P2X2 subunit (top), although it does not affect the amount of surface P2X3 (bottom). Histograms indicate changes in P2X2 (black) or P2X3 (white) surface receptors measured with optical density values expressed in AUs (n = 6 or 3 experiments, respectively; *p = 0.005) and normalized with respect to the protein amount in control condition (dashed line). D, Confocal microscopy photographs of TG neurons in the control and after anti-NGF treatment show different distribution of P2X2 immunostaining. In the absence of NGF, TG neurons express P2X2 receptors on the cell surface or close by (2.5 ± 1.5 μm beneath the surface; n = 15). Scale bar, 10 μm. Histograms show the relative percentage of neurons showing diffused or ring-like P2X2 immunoreactivity in control or after anti-NGF treatment (n = 469 or 211, respectively; *p < 0.001). E, Immunopurification of heteromeric P2X2/3 receptors was obtained with chemical cross-linking treatment (DTSSP) of membrane proteins. TG neuron extracts are immunoprecipitated (IP) with anti-P2X3 antibody and immunoblotted using anti-P2X2 antibody (lanes 1, 2). P2X2 subunit (64 kDa) is immunopurified from anti-NGF antibody-treated extracts (lane 2, arrowhead) and not from control (lane 1; n = 5 experiments) after DTSSP cross-linking. Anti-NGF-treated extracts, without DTSSP cross-linking, do not show any P2X2 signal (lane 3). P2X2 immunoprecipitation detects a single band (performed as control; lane 4, arrowhead). WB, Western blot. F, Anti-NGF treatment increases the fraction of TG neurons double immunopositive for P2X2 and P2X3 subunits (*p = 0.023), whereas chronic NGF application does not change this value with respect to control (n = 4).
