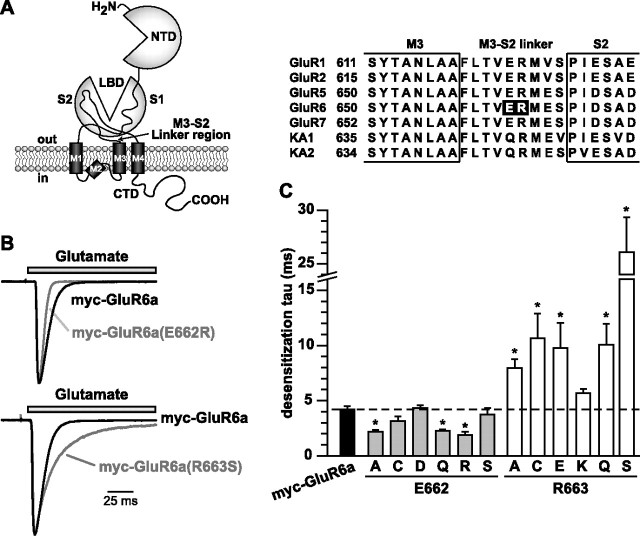
Figure 1.
Mutation of E662 and R663 in the myc-GluR6a subunit had opposite effects on kinetics of desensitization. A, Illustration of the domain structure of kainate receptors and other ionotropic glutamate receptors; the arrow indicates the region that contains the residues studied in this report. NTD, N-terminal domain; LBD, ligand-binding domain; M, membrane domains; CTD, C-terminal domain. An alignment of M3–S2 linker sequences in selected AMPA and kainate receptor subunits is also shown, with our mutation sites in the black background. B, Examples of glutamate-evoked currents from myc-GluR6a and myc-GluR6a(E662R) (top) or GluR6a(R663S) (bottom) receptors. Receptors were expressed in HEK 293-T/17 cells and patch-clamp recordings were performed at a holding potential of −70 mV. Glutamate (10 mm) was rapidly applied for 100 ms. Sample traces from the mutant receptors were scaled to the peak amplitude of myc-GluR6a currents to illustrate the difference in desensitization rates. C, Desensitization τ values derived from single-exponential fits to the current decay in the presence of glutamate for all the receptors in this study. Myc-GluR6a(R663S) was best fit with two exponential compounds; the value shown is a mean τ weighted for the contribution of both exponential decay components. Wild-type myc-GluR6a receptors desensitized with a τdes of 4.2 ± 0.2 ms are shown. The τ values of E662- and R663-substituted mutants were 2.1–4.3 and 5.6–26.1 ms, respectively (n = 3–7; *p < 0.05). Application of glutamate to myc-GluR6a(R663E) receptors failed to elicit a current in 26 of 29 transfected cells. Data represent mean ± SEM; individual values are given in supplemental Table 1 (available at www.jneurosci.org as supplemental material).