Figure 5.
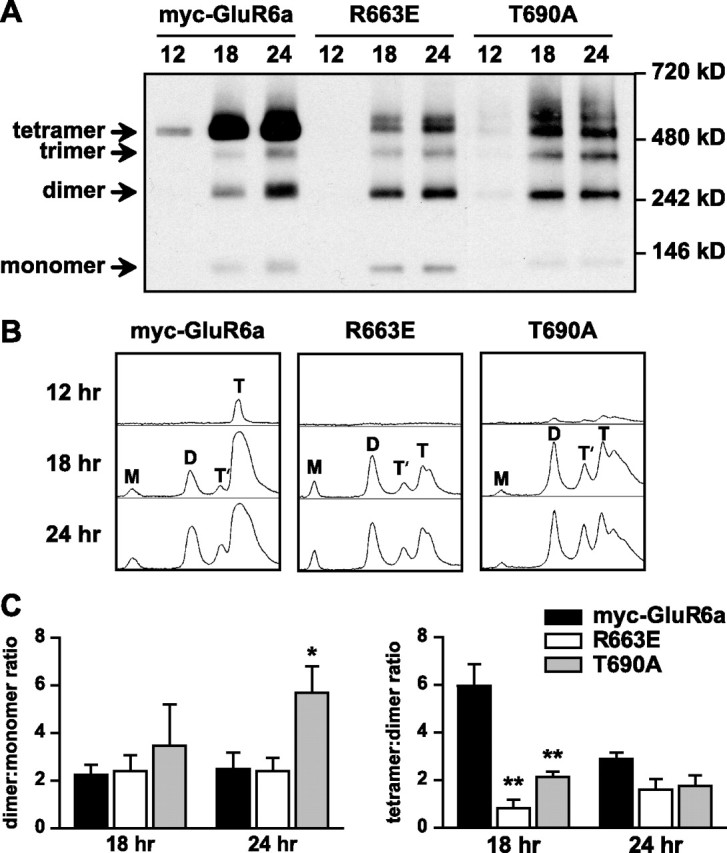
Reduced efficiency of tetrameric assembly of GluR6a receptor subunits mutated at both the M3–S2 linker and the ligand-binding domain. A, Nondenaturing PAGE and Western blots for anti-myc immunoreactivity were used to assess the assembly states of myc-GluR6a, GluR6a(R663E) (linker mutant), and GluR6a(T690A) (ligand-binding mutant) receptors at relatively early time points (12, 18, and 24 h) after transfection of COS-7 cells. Wild-type myc-GluR6a receptors were predominantly observed as tetramers at all time points after transfection. In contrast, the dimeric form was predominant for R663 at 18 h after transfection. T690A also showed significantly lower proportional representation of the tetrameric structure at the 18 h time point. B, Densitometric line scans of band densities shown in Figure 5 A. The areas under each peak were integrated to determine the percentage of monomers (M), dimers (D), trimers (T′), and tetramers (T). C, Left, Dimer/monomer ratios of integrated areas from densitometric scans were not significantly different at 18 h posttransfection, but at 24 h the T690A mutant showed a larger proportion of dimers (5.7 ± 1.1 vs 2.5 ± 0.7 for myc-GluR6a; n = 3; *p < 0.05). Right, Tetramer/dimer ratios of integrated areas from densitometric scans for myc-GluR6a(R663E) and T690A (0.8 ± 0.4 and 2.1 ± 0.2, respectively, n = 3) were greatly reduced compared with wild-type receptors at 18 h after transfection (6.0 ± 1.0; **p < 0.01). No difference in this ratio was apparent at 24 h posttransfection. Data represent mean ± SEM.
