Figure 7.
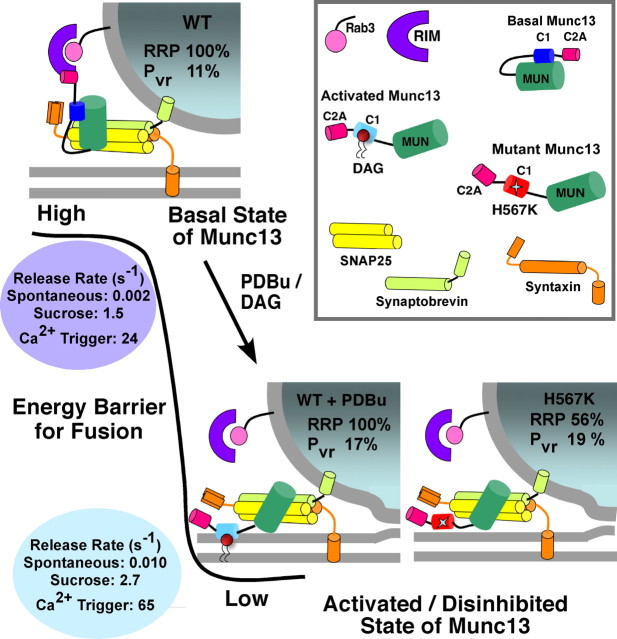
A two-state model for Munc13 activity. In the naive wild-type synapses, Munc13 exists in a folded configuration (top) in which intramolecular interactions between the N and C termini sterically interfere with the catalytic MUN domain. Such a basal “inhibited” state of Munc13 limits the vesicular release probability and rates by maintaining the energy barrier for fusion at a high level (shown in black). Interaction of Munc13 C2A with the RIM/Rab3 complex may have a stabilizing effect on the priming state. During binding of C1 domain with DAG/PDBu, the Munc13 molecule undergoes a conformational change that disrupts the intramolecular interactions and releases the catalytic domain from its inhibited condition to an activated state. The activation of Munc13 lowers the energy threshold for vesicular fusion, resulting in a potentiation of the kinetics of all forms of Ca2+ -dependent and -independent release. This process of enhancing the fusion willingness of vesicles comes at a stage after priming, possibly via downstream interactions of the MUN domain and the SNARE complex, and therefore impacts fusion probability without changing the RRP size. The Munc13-1H567K mutation leads to release probability and release rates in neurons that mimic the values observed in the C1 domain-activated WT, strongly suggesting that this gain-of-function mutation is a conformational mimic of the disinhibited “activated” state of Munc13. The constitutively open conformation may also have a negative impact on the upstream RIM/Rab3 interaction that normally helps in vesicle priming.