Figure 1.
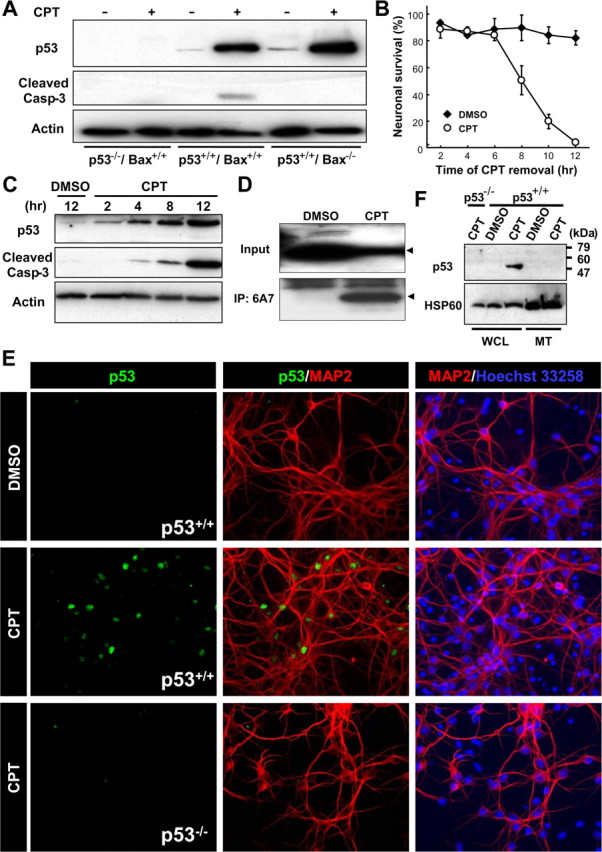
Endogenous p53 accumulates exclusively in the nuclear but not in the mitochondrial fraction during the commitment phase for camptothecin-induced neuronal apoptosis. A, Protein samples were prepared from p53+/+/Bax+/+, p53−/−/Bax+/+, or p53+/+/Bax−/− neurons treated with DMSO (vehicle control) or CPT (2.5 μm) for 12 h and analyzed for p53 protein expression (1C12 antibody) and cleaved caspase-3 by Western blotting. β-Actin was used as an internal loading control. B, Wild-type neurons were treated with CPT at 0 h and removed at the indicated times. Neuronal survival was assessed 24 h after exposure to CPT as described previously (Xiang et al., 1996) and is presented as the mean ± SD of triplicate cultures. C, Protein samples were prepared from wild-type neurons treated with DMSO or CPT for the indicated times and analyzed for expression of the p53 protein (1C12 antibody) and cleaved caspase-3 by Western blotting. β-Actin was used as an internal loading control. D, Wild-type neurons treated with CPT for 12 h were lysed in 1% CHAPS buffer. For analysis of the presence of activated Bax protein, the extracts were subjected to immunoprecipitation (IP) with anti-Bax (6A7) antibody, followed by Western blot analysis using anti-Bax (N-20) antibody. Arrowheads indicate the bands corresponding to the Bax protein. E, Wild-type neurons treated with CPT for 12 h were fixed and stained for the p53 protein (1C12 antibody) (green) and a neuronal marker microtubule-associated protein 2 (MAP2) (red), combined with nuclear staining with Hoechst 33258 (blue). Note that p53 immunoreactivity is observed exclusively in nuclei. p53−/− neurons do not display a fluorescent signal with the 1C12 antibody, demonstrating the specificity of the p53 immunoreactivity. Extranuclear accumulation of p53 was not observed in p53+/+ neurons treated with CPT for 4 or 8 h (data not shown). F, Whole-cell lysates (WCL) and mitochondrial fractions (MT) were prepared from p53+/+ or p53−/− neurons treated with CPT (2.5 μm) for 12 h and analyzed for p53 protein expression by Western blotting. Heat-shock protein 60 (HSP60) was used as a mitochondrial marker.
