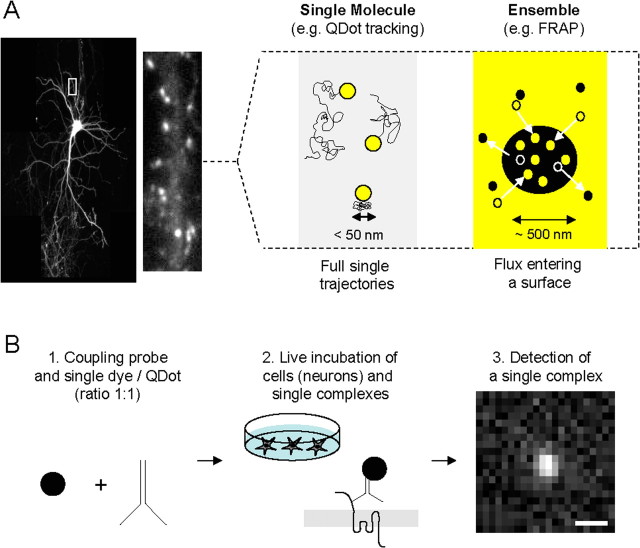
Figure 1.
Schematic description of single-molecule/particle experiment for the tracking of surface receptors in neurons. A, The first choice to make when planning to study receptors at the surface of neurons (left, hippocampal neuron expressing GFP-Homer 1C) is the type of experimental approach to setup. Right, There are schematically two ways to examine surface trafficking of receptors: ensemble or single-molecule approaches. The first one measures the surface behavior of the ensemble of labeled receptors using, for instance, FRAP. The second one measures the surface behavior of one (or possibly two if using antibody) receptor using single-molecule or Dot tracking. Although both approaches provide good measurements of receptor surface diffusion, differences in time and spatial resolutions exist. For instance, the spatial resolution in the type of microscopy used for FRAP and Dot experiments is defined by the diffraction limit, typically on the order of 200–300 nm. The single-molecule technique provides, however, a 10 nm pointing accuracy, which provides a unique way to collect the number of points necessary to build a trajectory in submicrometer membrane domains (e.g., synapse, lipid raft). B, Experimental procedure to run single-molecule or Dot tracking. First, the label used to detect the molecule of interest (e.g., antibody) is coupled to the tag (single dye, single Dot) in the ratio 1:1 (left). Second, live neurons are incubated with the single tag complexes (high dilution, ∼1 ng/ml) for several minutes. Third, single tag complexes are detected and tracked (see Single dye tracking and QDot tracking) over time as exemplified by the detection of a single Dot on a 30 ms acquisition image using a CCD camera. Scale bar, 600 nm. To ensure that the tracked complexes are in majority at the surface, rates of endocytosis of the receptor of interest should be first measured. SDT can then be performed during a time window in which only a low percentage (classically set as <15–20%) of receptors are internalized. For example, AMPAR surface tracking is performed only during 20 min after labeling, ensuring that <10–20% of labeled receptors are internalized. Note that the newly internalized AMPARs are mostly immobile and represent a small fraction of the single-molecule signals (Tardin et al., 2003).